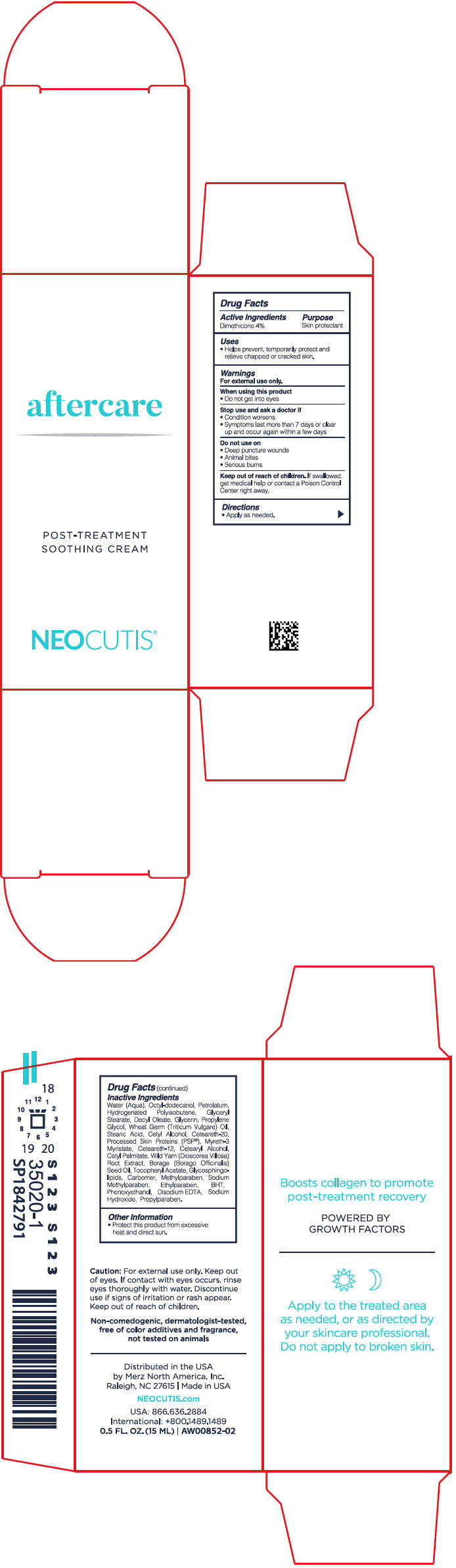
AFTER CARE- dimethicone cream
AFTER CARE by
Drug Labeling and Warnings
AFTER CARE by is a Otc medication manufactured, distributed, or labeled by Merz North America. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
- Uses
- Warnings
- Directions
-
Inactive ingredients
Water (Aqua), Octyl-Dodecanol, Petrolatum, Hydrogenated Polyisobutene, Glyceryl Stearate, Decyl Oleate, Glycerin, Propylene Glycol, Wheat Germ (Triticum Vulgare) Oil, Stearic Acid, Cetyl Alcohol, Ceteareth-20, Processed Skin Proteins (PSP(R)), Myreth-3 Myristate, Ceteareth-12, Cetearyl Alcohol, Cetyl Palmitate, Wild Yam (Dioscorea Villosa) Root Extract, Borage (Borago Officinalis) Seed Oil, Tocopheryl Acetate, Glycosphingolipids, Carbomer, Methylparaben, Sodium Methylparaben, Ethylparaben, BHT, Phenoxyethanol, Disodium EDTA, Sodium Hydroxide, Propylparaben.
- Other information
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 15 ML Bottle Carton
-
INGREDIENTS AND APPEARANCE
AFTER CARE
dimethicone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 46783-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 4 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) OCTYLDODECANOL (UNII: 461N1O614Y) PETROLATUM (UNII: 4T6H12BN9U) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) DECYL OLEATE (UNII: ZGR06DO97T) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WHEAT GERM OIL (UNII: 14C97E680P) STEARIC ACID (UNII: 4ELV7Z65AP) CETYL ALCOHOL (UNII: 936JST6JCN) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) HUMAN SKIN PROTEINS, PARTIALLY HYDROLYZED (UNII: G9813R29TW) MYRETH-3 MYRISTATE (UNII: O2C2MN32O6) CETEARETH-12 (UNII: 7V4MR24V5P) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETYL PALMITATE (UNII: 5ZA2S6B08X) DIOSCOREA VILLOSA TUBER (UNII: IWY3IWX2G8) BORAGE SEED OIL (UNII: F8XAG1755S) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) CARBOXYPOLYMETHYLENE (UNII: 0A5MM307FC) METHYLPARABEN (UNII: A2I8C7HI9T) METHYLPARABEN SODIUM (UNII: CR6K9C2NHK) ETHYLPARABEN (UNII: 14255EXE39) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) PHENOXYETHANOL (UNII: HIE492ZZ3T) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) SODIUM HYDROXIDE (UNII: 55X04QC32I) PROPYLPARABEN (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 46783-004-15 1 in 1 CARTON 04/01/2019 1 15 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC: 46783-004-20 1 in 1 CARTON 04/01/2019 2 200 mL in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC: 46783-004-04 4 mL in 1 TUBE; Type 0: Not a Combination Product 06/11/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 04/01/2019 Labeler - Merz North America (028147846)
Trademark Results [AFTER CARE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 AFTER CARE 75939929 2944153 Live/Registered |
Richards Homewares, Inc. 2000-03-09 |
 AFTER CARE 75545812 2349504 Dead/Cancelled |
PARTNERS IN HEALTH AND ENTERTAINMENT 1998-09-01 |
 AFTER CARE 75498741 not registered Dead/Abandoned |
AFTER CARE MEDICAL EQUIPMENT, INC. 1998-06-08 |
 AFTER CARE 75436348 not registered Dead/Abandoned |
XOMED SURGICAL PRODUCTS, INC. 1998-02-18 |
 AFTER CARE 73158733 1140157 Dead/Cancelled |
Physicians Formula Cosmetics, Inc. 1978-02-14 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.