TRANEXAMIC ACID IN SODIUM CHLORIDE- tranexamic acid injection, solution
TRANEXAMIC ACID IN SODIUM CHLORIDE by
Drug Labeling and Warnings
TRANEXAMIC ACID IN SODIUM CHLORIDE by is a Prescription medication manufactured, distributed, or labeled by Puniska Healthcare Private Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TRANEXAMIC ACID IN 0.7% SODIUM CHLORIDE INJECTION safely and effectively. See full prescribing information for TRANEXAMIC ACID IN 0.7% SODIUM CHLORIDE INJECTION.
TRANEXAMIC ACID IN 0.7% SODIUM CHLORIDE injection, for intravenous use
Initial U.S. Approval: 1986INDICATIONS AND USAGE
Tranexamic acid in 0.7% sodium chloride injection is an antifibrinolytic indicated in patients with hemophilia for short-term use (two to eight days) to reduce or prevent hemorrhage and reduce the need for replacement therapy during and following tooth extraction. (1)
DOSAGE AND ADMINISTRATION
- Before Extraction: Administer 10 mg/kg actual body weight of tranexamic acid in 0.7% sodium chloride injection intravenously with replacement therapy.
- After Extraction: Administer 10 mg/kg actual body weight 3 to 4 times daily for 2 to 8 days. Infuse no more than 10 mL/minute to avoid hypotension. (2.1)
DOSAGE FORMS AND STRENGTHS
Injection: 1,000 mg of tranexamic acid in 100 mL (10 mg/mL) sterile, unpreserved, clear, colorless solution in a single-dose bag for intravenous use. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Risk of thrombosis with concomitant use of Factor IX: Avoid concomitant use. (5.1)
- Seizures: Inadvertent injection into neuraxial system may result in seizures. (5.2)
- Hypersensitivity Reactions: In case of severe reaction, discontinue use and seek immediate medical attention. (5.3)
- Visual Disturbances: Visual or ocular adverse effects may occur. Discontinue use if visual or ocular symptoms occur. (5.4)
- Dizziness. Advise patients not to drive if dizziness occurs. (5.5)
ADVERSE REACTIONS
Most common adverse reactions are nausea, vomiting, diarrhea, allergic dermatitis, giddiness, hypotension and thromboembolic events. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Amneal Pharmaceuticals at 1-877-835-5472 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Recommended Dosage for Patients with Varying Degrees of Renal Impairment*
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Thromboembolic Risk
5.2 Seizures
5.3 Hypersensitivity Reactions
5.4 Visual Disturbances
5.5 Dizziness
6 ADVERSE REACTIONS
6.2 Post-marketing Experience
7 DRUG INTERACTIONS
7.1 Prothrombotic Medical Products
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dose of tranexamic acid in 0.7% sodium chloride injection is 10 mg/kg actual body weight intravenously administered as a single-dose, immediately before tooth extractions. Infuse no more than 10 mL/minute to avoid hypotension [see Warnings and Precautions (5.1)]. Following tooth extraction, tranexamic acid in 0.7% sodium chloride injection may be administered for 2 to 8 days at a dose of 10 mg/kg actual body weight three to four times daily, intravenously.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use tranexamic acid in 0.7% sodium chloride injection if particulate matter or coloration is seen.
Tranexamic acid in 0.7% sodium chloride injection should NOT be mixed with blood. The drug is a synthetic amino acid and should NOT be mixed with solutions containing penicillin.
The premix non-PVC bag contains no preservative; discard any unused portion.
2.2 Recommended Dosage for Patients with Varying Degrees of Renal Impairment*
For patients with moderate to severe impaired renal function, the following dosages are recommended:
Table 1: Recommended Dosage in Patients with Varying Degrees of Renal Impairment
Serum Creatinine (mg/dL)
Tranexamic Acid in 0.7% Sodium Chloride Injection Intravenous Dosage
1.36 to 2.83
(120 to 250 micromol/L)
10 mg/kg twice daily
2.83 to 5.66
(250 to 500 micromol/L)
10 mg/kg daily
> 5.66
(> 500 micromol/L)
10 mg/kg every 48 hours or 5 mg/kg every 24 hours
*Dose reduction is recommended for all doses, both before and after tooth extraction.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Tranexamic acid in 0.7% sodium chloride injection is contraindicated:
- In patients with subarachnoid hemorrhage. Anecdotal experience indicates that cerebral edema and cerebral infarction may be caused by tranexamic acid in such patients.
- In patients with active intravascular clotting [see Warnings and Precautions (5.1)].
- In patients with hypersensitivity to tranexamic acid or any of the ingredients [see Warnings and Precautions (5.3)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Thromboembolic Risk
Tranexamic acid in 0.7% sodium chloride injection is contraindicated in patients with active intravascular clotting.
Tranexamic acid is an antifibrinolytic and may increase the risk of thromboembolic events. Venous and arterial thrombosis or thromboembolism has been reported in patients treated with tranexamic acid. Avoid concomitant use of tranexamic acid in 0.7% sodium chloride injection and medical products that are pro-thrombotic, as the risk of thrombosis may be increased. These medications include but are not limited to, Factor IX Complex concentrates, Anti-inhibitor coagulant concentrates and hormonal contraceptives [see Drug Interactions (7.1), Use in Specific Populations (8.3)].
5.2 Seizures
Tranexamic acid may cause seizures, including focal and generalized seizures. The most common setting for tranexamic acid-induced seizures has been during cardiovascular surgery (a setting in which tranexamic acid in 0.7% sodium chloride injection is not FDA approved and which uses doses of up to ten-fold higher than the recommended human dose and in patients inadvertently given tranexamic acid into the neuraxial system). Tranexamic acid in 0.7% sodium chloride injection is not approved and not recommended for neuraxial administration. Consider dose reduction during surgery and dose adjustments for patients with clinical conditions such as renal dysfunction. Closely monitor the patient during surgery. Consider electroencephalogram (EEG) monitoring for patients with history of seizures or who experience myoclonic movements, twitching, or show evidence of focal seizures. Discontinue tranexamic acid in 0.7% sodium chloride injection if seizures occur.
5.3 Hypersensitivity Reactions
Cases of hypersensitivity reactions, including anaphylactic reactions, have occurred with use of intravenous tranexamic acid. Discontinue treatment with tranexamic acid in 0.7% sodium chloride injection if serious reaction occurs, provide appropriate medical management and do not restart treatment. Tranexamic acid in 0.7% sodium chloride injection is contraindicated in patients with a history of hypersensitivity to tranexamic acid.
5.4 Visual Disturbances
Although not seen in humans, focal areas of retinal degeneration have been observed in cats and dogs following oral or intravenous tranexamic acid at doses between 250 to 1,600 mg/kg/day (1.6 to 22 times the recommended usual human dose based on body surface area) from 6 days to 1 year. No retinal changes have been observed in eye examinations of patients treated with tranexamic acid for up to 8 years. Patients expected to be treated for greater than 3 months may consider ophthalmic monitoring including visual acuity and optical coherence tomography at regular intervals. Discontinue tranexamic acid in 0.7% sodium chloride injection if changes in ophthalmological examination occurs.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Thromboembolic Risk [see Warnings and Precautions (5.1)]
- Seizures [see Warnings and Precautions (5.2)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.3)]
- Visual Disturbances [see Warnings and Precautions (5.4)]
- Dizziness [see Warnings and Precautions (5.5)]
6.2 Post-marketing Experience
The following adverse reactions have been identified during post-approval use of tranexamic acid. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Gastrointestinal disturbances (nausea, vomiting, diarrhea) may occur and may resolve with dose-reduction. Allergic dermatitis and giddiness have been reported. Hypotension has been reported when intravenous injection is too rapid.
Thromboembolic events (e.g., deep vein thrombosis, pulmonary embolism, cerebral thrombosis, acute renal cortical necrosis and central retinal artery, vein obstruction and cases associated with concomitant use of combination hormonal contraceptives) have been rarely reported in patients receiving tranexamic acid for indications other than hemorrhage prevention in patients with hemophilia. Convulsion, cromatopsia and visual impairment have also been reported.
Anaphylaxis or anaphylactoid reactions have been reported that are suggestive of a causal relationship.
-
7 DRUG INTERACTIONS
7.1 Prothrombotic Medical Products
Avoid concomitant use of tranexamic acid in 0.7% sodium chloride injection with medical products that are prothrombotic because concomitant use can further increase the risk of thromboembolic adverse reactions associated with tranexamic acid [see Warnings and Precautions (5.1), Use in Specific Populations (8.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from published studies, case series and case reports with tranexamic acid use in pregnant women in the second and third trimester and at the time of delivery have not clarified whether there is a drug-associated risk of miscarriage or adverse maternal or fetal outcomes. There are 2 (0.02%) infant cases with structural abnormalities that resulted in death when tranexamic acid was used during conception or the first trimester of pregnancy; however, due to other confounding factors the risk of major birth defects with use of tranexamic acid during pregnancy is not clear. Tranexamic acid is known to pass the placenta and appears in cord blood at concentrations approximately equal to maternal concentration (see Data).
Reproduction studies performed in mice, rats and rabbits have not revealed any adverse effects on the fetus due to tranexamic acid administered during organogenesis. Doses examined were multiples of up to 3 times (mouse), 6 times (rat) and 3 times (rabbit) the maximum human dose based on body surface area in the mouse, rat and rabbit, respectively (see Data).
The estimated background risk for major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in the clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
It is not known whether tranexamic acid use in pregnant women may cause a drug-associated risk of miscarriage or adverse maternal or fetal outcomes. For decisions regarding the use of tranexamic acid in 0.7% sodium chloride injection during pregnancy, the potential risk of tranexamic acid in 0.7% sodium chloride injection administration on the fetus should always be considered along with the mother’s clinical need for tranexamic acid in 0.7% sodium chloride injection; an accurate risk-benefit evaluation should drive the treating physician’s decision.
Data
Human Data
Tranexamic acid passes through the placenta. The concentration in cord blood after an intravenous injection of 10 mg/kg to pregnant women is about 30 mg/L, as high as in the maternal blood.
There were 13 clinical studies that described fetal and/or neonatal functional issues such as low Apgar score, neonatal sepsis, cephalohematoma and 9 clinical studies that discussed alterations to growth including low birth weight and preterm birth at 22 to 36 weeks of gestation in fetuses and infants exposed to tranexamic acid in-utero.
Animal Data
In embryo-fetal development studies, tranexamic acid was administered to pregnant mice from Gestation day (GD) 6 through GD 12 and rats from GD 9 through GD 14 at daily doses of 0.3 g/kg or 1.5 g/kg. There was no evidence of adverse developmental outcomes in mice and rats at multiple of 3 and 6 times the maximum recommended human dose based on body surface area in the mouse and rat, respectively.
In rabbits, tranexamic acid was administered intravenously at doses of 50, 100, or 200 mg/kg/day or orally at doses of 100, 200, or 400 mg/kg/day from GD 6 through GD 18. There was no evidence of adverse developmental outcomes at dose multiples of 2 or 3 times, respectively, the maximum recommended human dose based on body surface area. Intravenous doses of 200 mg/kg/day showed slightly retarded weight gain in pregnant rabbits.
8.2 Lactation
Risk Summary
Published literature reports the presence of tranexamic acid in human milk. There are no data on the effects of tranexamic acid on the breastfed child or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for tranexamic acid in 0.7% sodium chloride injection and any potential adverse effects on the breastfed child from tranexamic acid in 0.7% sodium chloride injection or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Contraception
Concomitant use of tranexamic acid in 0.7% sodium chloride injection, which is an antifibrinolytic, with hormonal contraceptives may increase the risk for thromboembolic adverse reactions. Advise patients to use an effective alternative (nonhormonal) contraceptive method [see Warnings and Precautions (5.5), Drug Interactions (7.1)].
8.4 Pediatric Use
There are limited data concerning the use of tranexamic acid in pediatric patients with hemophilia who are undergoing tooth extraction. The limited data suggest that there are no significant pharmacokinetic differences between adult and pediatric patients.
8.5 Geriatric Use
Clinical studies of tranexamic acid did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
This drug is known to be substantially excreted by the kidney and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection and it may be useful to monitor renal function [Dosage and Administration (2.2), Clinical Pharmacology (12.3)].
8.6 Renal Impairment
Reduce the dosage of tranexamic acid in 0.7% sodium chloride injection in patients with renal impairment, based on the patient’s serum creatinine [see Dosage and Administration (2.2), Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
Cases of overdosage of tranexamic acid have been reported. Based on these reports, symptoms of overdosage may be gastrointestinal, e.g., nausea, vomiting, diarrhea; hypotensive, e.g., orthostatic symptoms; thromboembolic; e.g., arterial, venous, embolic; neurologic, e.g., visual impairment, convulsions, headache, mental status changes; myoclonus; and rash. Tranexamic acid is not dialyzable.
-
11 DESCRIPTION
Tranexamic acid, USP is trans-4-(aminomethyl)cyclohexanecarboxylic acid, an antifibrinolytic agent. Tranexamic acid, USP is a white, crystalline powder. The structural formula is
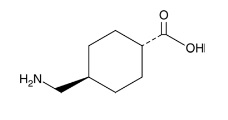
Molecular Formula: C8H15NO2 Molecular Weight: 157.21 g/mol
Tranexamic Acid in 0.7% Sodium Chloride Injection, 1,000 mg per 100 mL (10 mg per mL) is a sterile, clear, colorless, nonpyrogenic injectable solution for intravenous administration. Each IV bag contains: 1,000 mg of tranexamic acid, USP; 700 mg of sodium chloride, USP and water for injection, USP. The aqueous solution has a pH of 6.5 to 8.0.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Tranexamic acid is a synthetic lysine amino acid derivative, which diminishes the dissolution of hemostatic fibrin by plasmin. In the presence of tranexamic acid, the lysine receptor binding sites of plasmin for fibrin are occupied, preventing binding to fibrin monomers, thus preserving and stabilizing fibrin’s matrix structure.
The antifibrinolytic effects of tranexamic acid are mediated by reversible interactions at multiple binding sites within plasminogen. Native human plasminogen contains 4 to 5 lysine binding sites with low affinity for tranexamic acid (Kd = 750 μmol/L) and 1 with high affinity (Kd = 1.1 μmol/L). The high affinity lysine site of plasminogen is involved in its binding to fibrin. Saturation of the high affinity binding site with tranexamic acid displaces plasminogen from the surface of fibrin. Although plasmin may be formed by conformational changes in plasminogen, binding to and dissolution of the fibrin matrix is inhibited.
12.2 Pharmacodynamics
Tranexamic acid in concentrations of 1 mg/mL and 10 mg/mL prolongs the thrombin time. An antifibrinolytic concentration of tranexamic acid remains in different tissues for about 17 hours and in the serum, up to seven or eight hours.
Tranexamic acid in concentrations up to 10 mg/mL blood has no influence on the platelet count, the coagulation time or various coagulation factors in whole blood or citrated blood from healthy subjects.
12.3 Pharmacokinetics
Distribution
The initial volume of distribution is about 9 liters to 12 liters. The plasma protein binding of tranexamic acid is about 3% at therapeutic plasma levels and seems to be fully accounted for by its binding to plasminogen. Tranexamic acid does not bind to serum albumin.
Elimination
After an intravenous dose of 1 g, the plasma concentration time curve shows a triexponential decay with a half-life of about 2 hours for the terminal elimination phase.
Excretion
Urinary excretion is the main route of elimination via glomerular filtration. Overall renal clearance is equal to overall plasma clearance (110 mL/min to 116 mL/min) and more than 95% of the dose is excreted in the urine as unchanged drug. Excretion of tranexamic acid is about 90% at 24 hours after intravenous administration of 10 mg/kg body weight.
Specific Populations
Patients with Renal Impairment
The blood levels of tranexamic acid are increased in patients with renal insufficiency. Urinary excretion following a single intravenous injection of tranexamic acid declines as renal function decreases. Following a single 10 mg/kg intravenous injection of tranexamic acid, the 24-hour urinary fractions of tranexamic acid with serum creatinine concentrations 1.4 to 2.8, 2.8 to 5.7 and greater than 5.7 mg/dL were 51, 39 and 19%, respectively. The 24-hour tranexamic acid plasma concentrations for these patients demonstrated a direct relationship to the degree of renal impairment. Therefore, dose adjustment is needed in patients with renal impairment [see Dosage and Administration (2.2), Use in Specific Populations (8.6)].
Drug Interaction Studies
No studies of interactions between tranexamic acid in 0.7% sodium chloride injection and other drugs have been conducted.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Tranexamic acid was not carcinogenic in a 2-year study in rats and mice at oral doses up to 3 and 5.3 g/kg/day, which are approximately 12 and 11 times the maximum recommended human dose based on body surface area, respectively. Tranexamic acid was not genotoxic in the reverse mutation bacterial (Ames) test and in vitro and in vivo cytogenetic test.
In a fertility and early embryonic development study, tranexamic acid was administered to male rats as 0.3% and 1% of drug in diet (average doses of 222 and 856 mg/kg/day) or to female rats at dose levels of 0.3% and 1.2% of drug in diet. Tranexamic acid had no effect on fertility or reproductive function of male or female rats at dose multiples of 4 or 5 times the maximum recommended human dose based on body surface area, respectively.
13.2 Animal Toxicology and/or Pharmacology
Nonclinical studies have shown a retinal toxicity associated with tranexamic acid. Toxicity is characterized by retinal atrophy commencing with changes to the retinal pigmented epithelium and progressing to retinal detachment in cats. The toxicity appears to be dose related and changes are partially reversible at lower doses. Effects were observed in dogs at oral doses of 800 mg/kg/day and higher (multiple of 11 times the maximum human dose based on body surface area) and in cats at 250 mg/kg/day for 14 days (multiple of 1.6 times the maximum human dose based on body surface area). Some fully reversible changes in pigmentation were observed in cats at doses of 125 mg/kg/day (multiple of 0.8 times the maximum human dose based on body surface area). Studies suggest that the underlying mechanism may be related to a transient retinal ischemia at high exposures, linked to the known sympathomimetic effect of high plasma exposures of tranexamic acid.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Tranexamic Acid in 0.7% Sodium Chloride Injection, 1,000 mg per 100 mL (10 mg per mL) is supplied as a sterile, unpreserved, clear, colorless solution in a single-dose polymeric bag containing 1,000 mg tranexamic acid in 100 mL of solution sealed with a Twist-off port and oversealed in an aluminum pouch.
It is available as follows:
Strength
Each
Unit of Sale
1,000 mg per 100 mL
(10 mg per mL)
NDC: 80830-2329-1
1 Single-dose Infusion Bag in an Overwrap
NDC: 80830-2329-2
Unit of 10
NDC: 80830-2329-3
Unit of 12Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Discard any unused portion.
-
17 PATIENT COUNSELING INFORMATION
Thromboembolic Risk
- Inform patients that tranexamic acid in 0.7% sodium chloride injection may increase the risk of venous and arterial thrombosis or thromboembolism and to contact their healthcare provider for any signs or symptoms suggestive of thromboembolism.
- Advise patients using hormonal contraception that combined use with tranexamic acid in 0.7% sodium chloride injection may increase the risk for thromboembolic adverse reactions and to use effective alternative (nonhormonal) contraception during therapy with tranexamic acid in 0.7% sodium chloride injection [see Warnings and Precautions (5.1), Drug Interactions (7.1), Use in Specific Populations (8.3)].
Seizures
Inform patients that tranexamic acid in 0.7% sodium chloride injection may cause seizures and to contact their healthcare provider for any signs or symptoms suggestive of seizures [see Warnings and Precautions (5.2)].
Hypersensitivity Reactions
Inform patients that tranexamic acid in 0.7% sodium chloride injection may cause hypersensitivity reactions and to contact their healthcare provider for any signs or symptoms of hypersensitivity reactions [see Warnings and Precautions (5.3)].
Visual Disturbances
Inform patients that tranexamic acid in 0.7% sodium chloride injection can cause visual disturbance and that they should report any eye symptoms or change in their vision to their healthcare provider and to follow-up with an ophthalmologist for a complete ophthalmologic evaluation, including dilated retinal examination of the retina [see Warnings and Precautions (5.4)].
Risk of Driving and Operating Machinery
Inform patients that tranexamic acid in 0.7% sodium chloride injection may cause dizziness and that the patient should be cautioned about driving, operating machinery, or performing hazardous tasks while taking tranexamic acid in 0.7% sodium chloride injection [see Warnings and Precautions (5.5)].
Manufactured by:
Amneal Pharmaceuticals Pvt. Ltd.
Parenteral Unit
Ahmedabad 382110, INDIA
Distributed by:
Amneal Pharmaceuticals LLC
Bridgewater, NJ 08807
Rev. 11-2023-02
-
PRINCIPAL DISPLAY PANEL
NDC: 80830-2329-1
Tranexamic Acid in 0.7% Sodium Chloride Injection
1,000 mg/100 mL (10 mg/mL)
Rx only
Intravenous Bag Label
Amneal Pharmaceuticals LLC

NDC: 80830-2329-1
Tranexamic Acid in 0.7% Sodium Chloride Injection
1,000 mg/100 mL (10 mg/mL)
Rx only
Pouch Label
Amneal Pharmaceuticals LLC

Pouch label
NDC: 80830-2329-2
Tranexamic Acid in 0.7% Sodium Chloride Injection
1,000 mg/100 mL (10 mg/mL)
Rx only
10 x 100 mL Carton Label
Amneal Pharmaceuticals LLC
Shipper label (10 x 100 mL)
NDC: 80830-2329-3
Tranexamic Acid in 0.7% Sodium Chloride Injection
1,000 mg/100 mL (10 mg/mL)
Rx only
12 x 100 mL Carton Label
Amneal Pharmaceuticals LLC

Shipper label (12 x 100 mL)
-
INGREDIENTS AND APPEARANCE
TRANEXAMIC ACID IN SODIUM CHLORIDE
tranexamic acid injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 80830-2329 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRANEXAMIC ACID (UNII: 6T84R30KC1) (TRANEXAMIC ACID - UNII:6T84R30KC1) TRANEXAMIC ACID 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 7 mg in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 80830-2329-2 10 in 1 CARTON 10/23/2023 1 1 in 1 POUCH 1 100 mL in 1 BAG; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC: 80830-2329-3 12 in 1 CARTON 01/08/2024 2 1 in 1 POUCH 2 100 mL in 1 BAG; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA217155 10/23/2023 Labeler - Amneal Pharmaceuticals Private Limited (675474666) Establishment Name Address ID/FEI Business Operations Amneal Pharmaceuticals Private Limited 675474666 analysis(80830-2329) , label(80830-2329) , manufacture(80830-2329) , pack(80830-2329) , sterilize(80830-2329)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.