ED CHLORPED D by EDWARDS PHARMACEUTICALS, INC. ED CHLORPED D
ED CHLORPED D by
Drug Labeling and Warnings
ED CHLORPED D by is a Otc medication manufactured, distributed, or labeled by EDWARDS PHARMACEUTICALS, INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ED CHLORPED D- chlorpheniramine maleate and phenylephrine hydrochloride liquid
EDWARDS PHARMACEUTICALS, INC.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
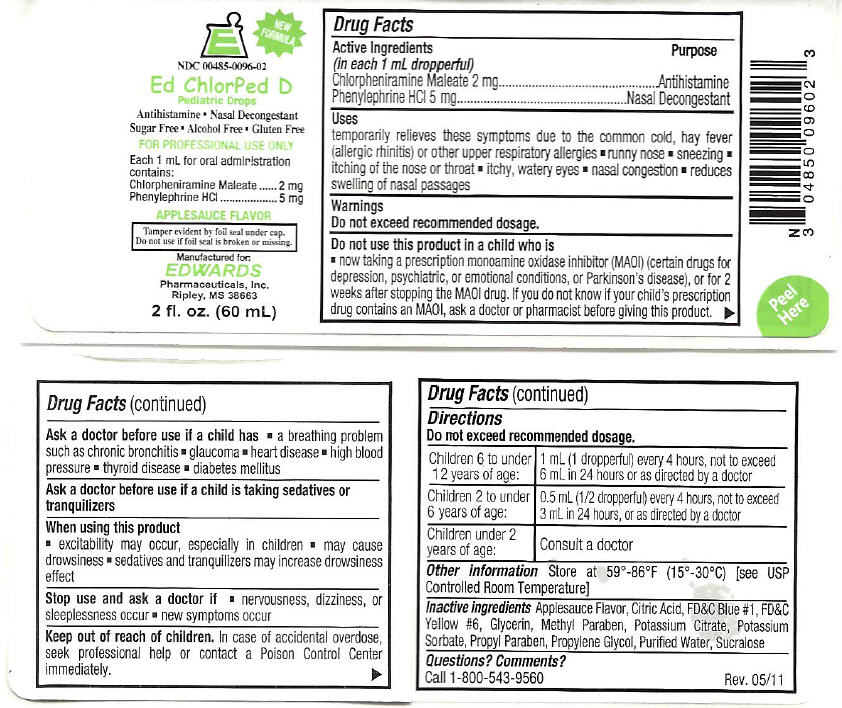
ED CHLORPED D
Uses
temporarily relieves these symptoms due to the common cold, hay fever (allergic rhinitis) or other upper respiratory allergies
- runny nose
- sneezing
- itching of the nose or throat
- itchy, watery eyes
- nasal congestion
- reduces swelling of nasal passages
Warnings
Do not exceed recommended dosage.
Do not use this product in a child who is
- now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your child's prescription drug contains an MAOI, ask a doctor or pharmacist before giving this product.
Ask a doctor before use if a child has
- a breathing problem such as chronic bronchitis
- glaucoma
- heart disease
- high blood pressure
- thyroid disease
- diabetes mellitus
Directions
Do not exceed recommended dosage.
| Children 6 to under 12 years of age: | 1 mL (1 dropperful) every 4 hours, not to exceed 6 mL in 24 hours or as directed by a doctor |
| Children 2 to under 6 years of age: | 0.5 mL (1/2 dropperful) every 4 hours, not to exceed 3 mL in 24 hours, or as directed by a doctor |
| Children under 2 years of age: | Consult a doctor |
Inactive ingredients
Applesauce Flavor, Citric Acid, FD&C Blue #1, FD&C Yellow #6, Glycerin, Methyl Paraben, Potassium Citrate, Potassium Sorbate, Propyl Paraben, Propylene Glycol, Purified Water, Sucralose
PRINCIPAL DISPLAY PANEL - 60 mL Bottle Label
E
NEW
FORMULA
NDC: 00485-0096-02
Ed ChlorPed D
Pediatric Drops
Antihistamine ▪ Nasal Decongestant
Sugar Free ▪ Alcohol Free ▪ Gluten Free
FOR PROFESSIONAL USE ONLY
Each 1 mL for oral administration
contains:
Chlorpheniramine Maleate
2 mg
Phenylephrine HCl
5 mg
APPLESAUCE FLAVOR
Tamper evident by foil seal under cap.
Do not use if foil seal is broken or missing.
Manufactured for:
EDWARDS
Pharmaceuticals, Inc.
Ripley, MS 38663
2 fl. oz. (60 mL)
| ED CHLORPED D
chlorpheniramine maleate and phenylephrine hydrochloride liquid |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - EDWARDS PHARMACEUTICALS, INC. (195118880) |