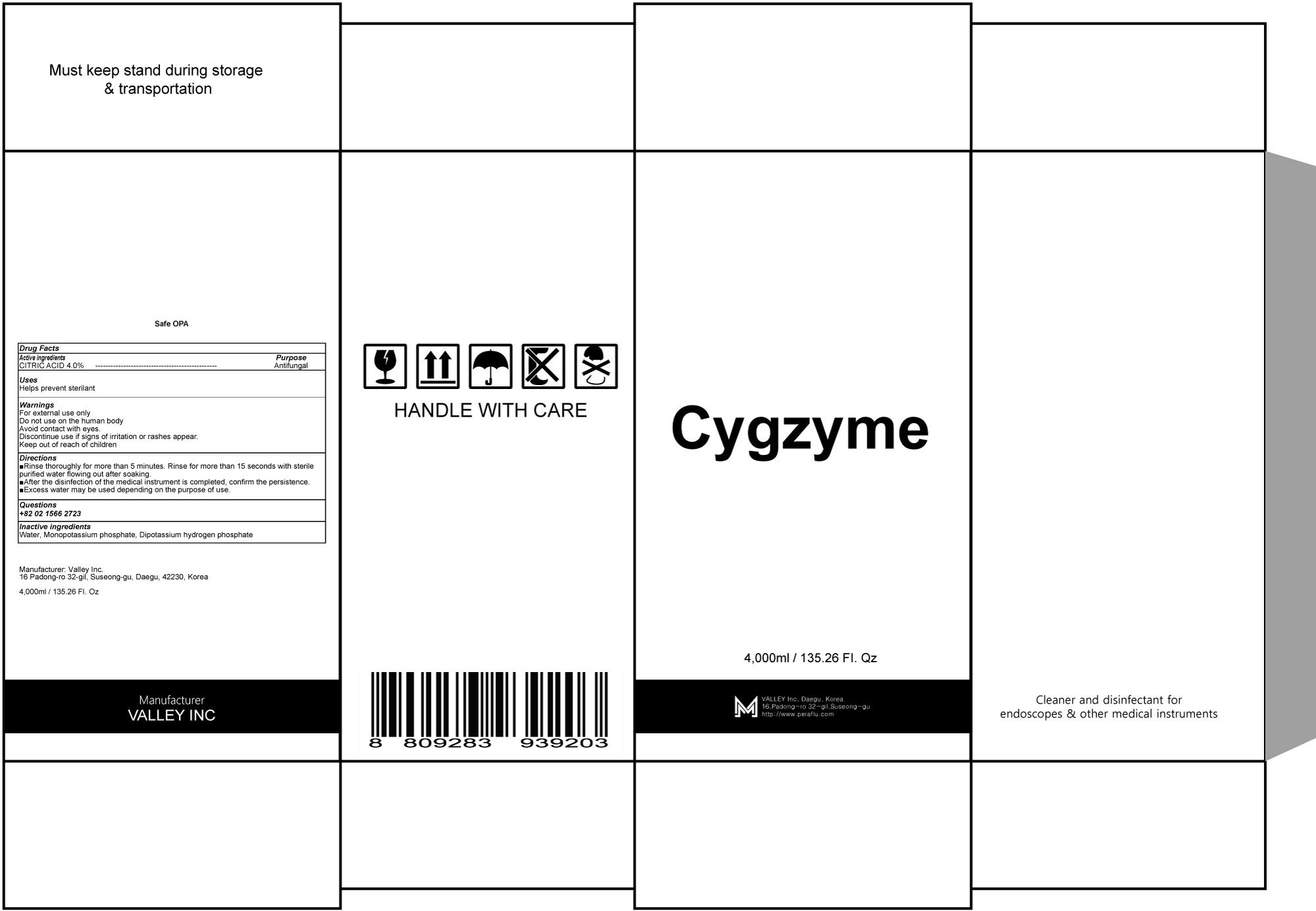
Cygzyme by Valley Inc.
Cygzyme by
Drug Labeling and Warnings
Cygzyme by is a Otc medication manufactured, distributed, or labeled by Valley Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CYGZYME- citric acid liquid
Valley Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
INACTIVE INGREDIENTS
Water, Sodium hydrogencarbonate, Gluconic acid, Sodium salt, N-Ethylenediaminediacetic acid,Dihydrogen Oxide
WARNINGS
For external use only
Do not use on the human body
Avoid contact with eyes.
Discontinue use if signs of irritation or rashes appear.
Keep out of reach of children
| CYGZYME
citric acid liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Valley Inc. (695095445) |
| Registrant - Valley Inc. (695095445) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Valley Inc. | 695095445 | manufacture(81803-040) | |
Revised: 3/2024
Document Id: 10dd95c1-ffe0-43e0-bbf2-9b6ca4e0d990
Set id: c1b0c21f-0b9a-4586-ac5c-02daa690249a
Version: 2
Effective Time: 20240312
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.