RYMED- dexchlorpheniramine maleate and phenylephrine hydrochloride tablet, coated
RYMED by
Drug Labeling and Warnings
RYMED by is a Otc medication manufactured, distributed, or labeled by EDWARDS PHARMACEUTICALS, INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
-
Warnings
- Do not exceed recommended dosage.
Do not use this product
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- heart disease
- high blood pressure
- thyroid disease
- diabetes mellitus
- difficulty in urination due to enlargement of the prostate gland
When using this product
- excitability may occur, especially in children
- may cause drowsiness
- alcohol, sedatives and tranquilizers may increase the drowsiness effect
- avoid alcoholic beverages
- use caution when driving a motor vehicle or operating machinery
-
Directions
Do not exceed recommended dosage.
Adults and children 12 years of age and over: 1 tablet every 4 hours, not to exceed 6 tablets in 24 hours, or as directed by a doctor Children 6 to under 12 years of age: 1/2 tablet every 4 hours, not to exceed 3 tablets in 24 hours, or as directed by a doctor Children under 6 years of age: Consult a doctor. - Inactive ingredients
- Questions or Comments?
-
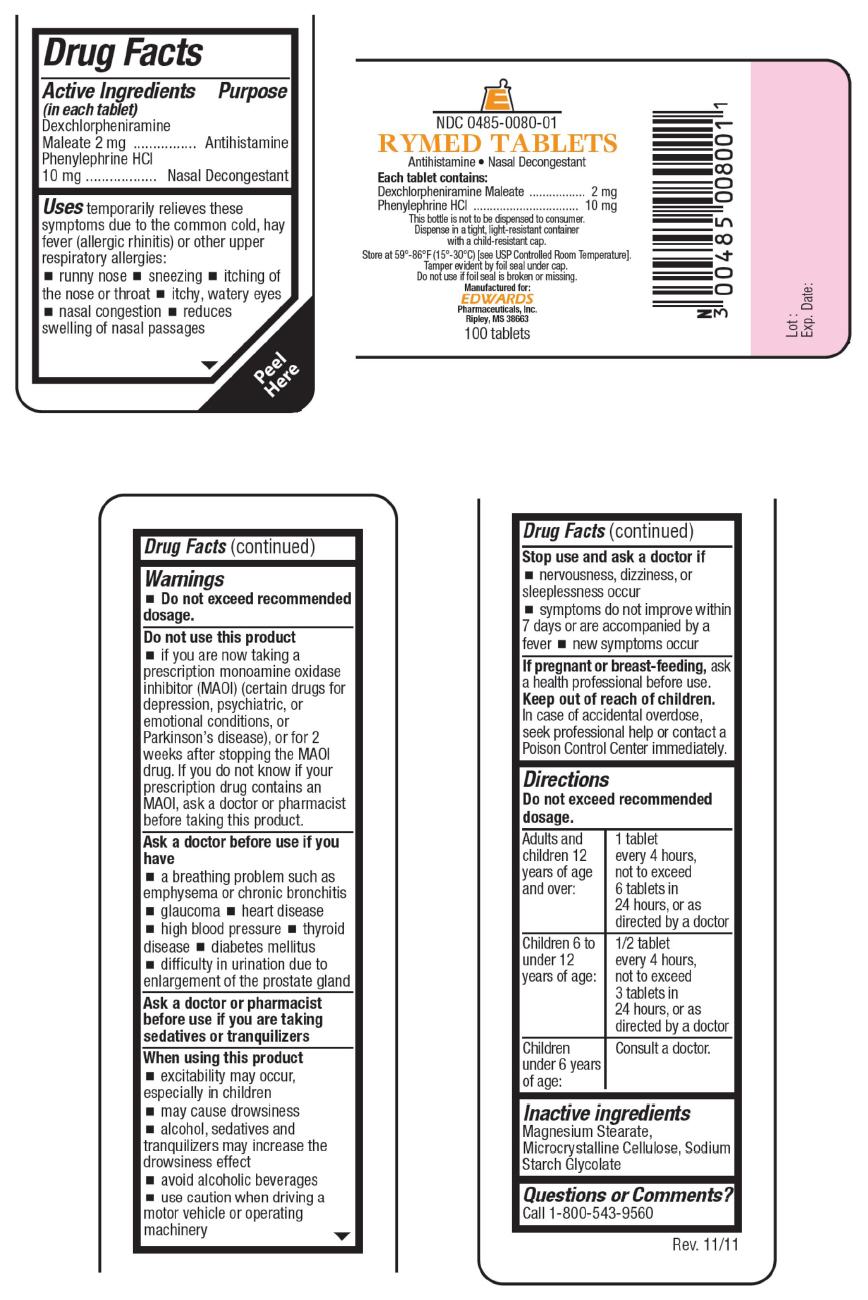
PRINCIPAL DISPLAY PANEL - 100 Tablet Bottle Label
E
NDC: 0485-0080-01
RYMED TABLETS
Antihistamine Nasal DecongestantEach tablet contains:
Dexchlorpheniramine Maleate 2 mg
Phenylephrine HCl 10 mg
This bottle is not to be dispensed to consumer.
Dispense in a tight, light-resistant container
with a child-resistant cap.Store at 59°-86° (15°-30°C) [see USP Controlled Room Temperature].
Tamper evident by foil seal under cap.
Do not use if foil seal is broken or missing.
Manufactured for:
EDWARDS
Pharmaceuticals, Inc.
Ripley, MS 38663100 tablets
-
INGREDIENTS AND APPEARANCE
RYMED
dexchlorpheniramine maleate and phenylephrine hydrochloride tablet, coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0485-0080 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXCHLORPHENIRAMINE MALEATE (UNII: B10YD955QW) (DEXCHLORPHENIRAMINE - UNII:3Q9Q0B929N) DEXCHLORPHENIRAMINE MALEATE 2 mg PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A CORN (UNII: AG9B65PV6B) Product Characteristics Color white Score 2 pieces Shape OVAL Size 12mm Flavor Imprint Code R;1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0485-0080-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/15/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 11/15/2011 Labeler - EDWARDS PHARMACEUTICALS, INC. (195118880)
Trademark Results [RYMED]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 RYMED 86281706 4656342 Live/Registered |
Edwards Pharmaceuticals, Inc. 2014-05-15 |
 RYMED 79194355 5234462 Live/Registered |
Rymed Pty Ltd 2016-07-19 |
 RYMED 73284393 not registered Dead/Abandoned |
EDWARD'S PHARMACAL, INC. 1980-11-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.