HYDRO 35® Hydrating Topical Foam
Hydro 35 by
Drug Labeling and Warnings
Hydro 35 by is a Prescription medication manufactured, distributed, or labeled by Exeltis USA Dermatology, LLC, Americal Spraytech. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
HYDRO 35- urea in a water and lipid based foam containing lactic acid aerosol, foam
Exeltis USA Dermatology, LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
HYDRO 35®
Hydrating Topical Foam
For Topical Dermatological Use Only
Rx Only - Caution: Federal Law restricts this product to sale by, or on the order of a licensed healthcare practitioner.
DESCRIPTION
HYDRO 35 Foam is a keratolytic agent delivered in a water & lipid based emollient foam containing lactic acid. This foam gently softens excess tissue to enhance removal from skin and nails, while rehydrating healthy tissue. Each gram contains 35% Urea as the active ingredient.
| CHEMICAL STRUCTURE | 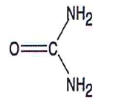 |
| Urea has the following chemical structure: |
CLINICAL PHARMACOLOGY
Topically applied urea dissolves the intercellular matrix of the skin which results in softening of the hyperkeratotic tissue, and thus enhances shedding of scaly, dry skin. Urea topically applied to the nail plate has a similar effect on the intercellular matrix of the nail plate.
INDICATIONS FOR USE
For enzyme debridernent and promotion of normal healing of surface lesions, particularly where healing is retarded by local infection. Necrotic tissue. fibrinous or purulent debris. Or eschar. Topically applied urea is useful for the treatment of hyperkeratotic conditions such as dermatitis. psoriasis, xerosis.ichthyosis, eczema.keratosis.keratoderma, and dry, rough skin, as well as corns and calluses and damaged, ingrown and devitalized nails.
CONTRAINDICATIONS
HYDRO 35 Foam should not be used by persons who have a known hypersensitivity to urea or any of the listed ingredients.
WARNINGS
For external use only. Not for ophthalmic, oral, and or intravaginal use. Avoid contact with the eyes, lips, and other mucous membranes.
KEEP THIS AND OTHER MEDICATIONS OUT OF THE REACH OF CHILDREN.
Contains flammable materials. Contents under pressure. Do not puncture or incinerate. Do not expose to temperatures over 120'F (48'C) even when empty.
PRECAUTIONS
Use only as directed by a healthcare practitioner. Do not use to treat any condition other than that for which it is prescribed. If redness or irritation occurs, discontinue use and contact prescribing healthcare practitioner.
Pregnancy (Category C)
Animal reproduction studies have not been conducted with Hydro 35 Foam. It is also not known whether Hydro 35 Foam can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Hydro 35 Foam should be given to a pregnant woman only if clearly needed.
DOSAGE AND ADMINISTRATION
Apply to affected area twice a day unless otherwise directed by a prescribing healthcare practitioner. HYDRO 35 Foam should be rubbed gently into the skin until it is completely absorbed.
Follow these important directions to ensure proper foaming and maximum delivery of product:
|  |
INGREDIENTS
Urea 35%, dimethicone, ethylparaben. Glycerin, Lactic acid, methyl paraben, phenoxyethanol, polysorbate 20.povidone, propylene glycol. propylparaben, purified water, Stearic acid, trolamine, and as propellants isobutane and propane.
HOW SUPPLIED
HYDRO 35 Foam is supplied in a 5.3 ounce (150g) pressurized canister bearing the NDC Number 23710-035-15 and i n a 0.79 ounce (22g) pressurized canister bearing the NDC Number 23710-035-20.
US Patent 5,993.830
Manufactured in the USA for
Exeltis USA Dermatology, LLC
Florham Park, NJ 07932
www.exeltisUSA.com
© 2012 Exeltis USA Dermatology, LLC
Rev 09/2015
51000020
PRINCIPAL DISPLAY PANEL - 150 g Canister Label
NDC: 23710-035-15
HYDRO 35®
35% UREA IN A VEHICLE
CONTAINING LACTIC ACID
HYDRATING TOPICAL FOAM
PRODERM TECHNOLOGY®
Rx Only
NET WT. 5.3 OZ (150 g)

PRINCIPAL DISPLAY PANEL - 150 g Canister Carton
NDC: 23710-035-15
HYDRO 35®
35% UREA IN A VEHICLE
CONTAINING LACTIC ACID
HYDRATING TOPICAL FOAM
PRODERM TECHNOLOGY®
Rx Only
NET WT. 5.3 OZ (150 g)

| HYDRO 35
urea in a water and lipid based foam containing lactic acid aerosol, foam |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - Exeltis USA Dermatology, LLC (078715346) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Americal Spraytech | 137135237 | MANUFACTURE(23710-035) , LABEL(23710-035) | |