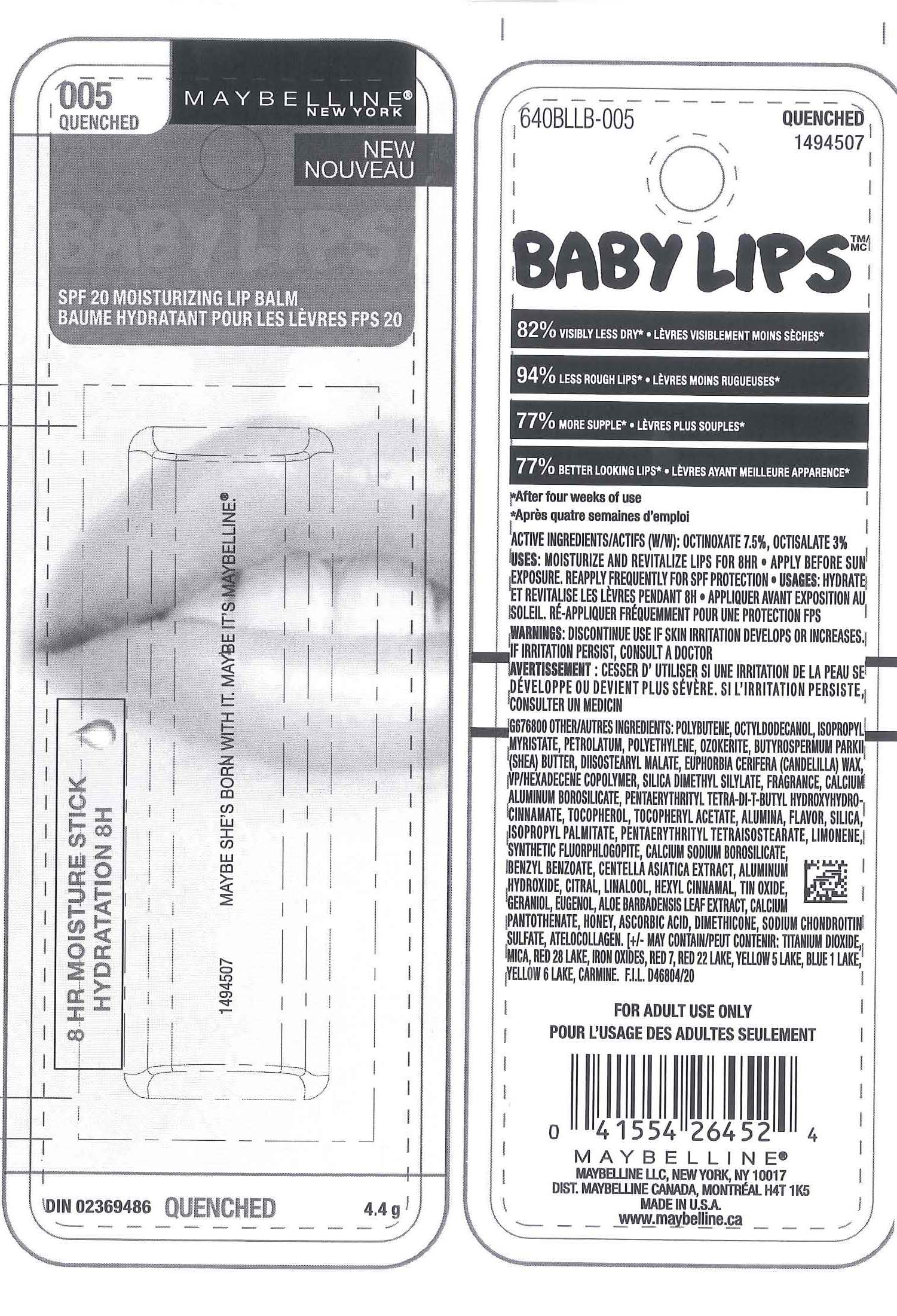
MAYBELLINE NEW YORK BABY LIPS MOISTURIZING LIP BALM SPF 20- octinoxate octisalate lipstick
Maybelline New York by
Drug Labeling and Warnings
Maybelline New York by is a Otc medication manufactured, distributed, or labeled by L'Oreal USA Products Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredients
- Uses
- Directions
- Warnings
-
Inactive ingredients
polybutene, octyldodecanol, isopropyl myristate, petrolatum, polyethylene, ozokerite, butyrospermum park II (shea) butter, diisostearyl malate, euphorbia cerifera (candelilla) wax, vp/hexadecene copolymer, silica dimethyl silylate, fragrance, calcium aluminum borosilicate, pentaerythrityl tetra-di-t-butyl hydroxyhydrocinnamate, tocopherol, tocopheryl acetate, alumina, flavor, silica, isopropyl palmitate, pentaerythrityl tetraisostearate, limonene, synthetic fluorphlogopite, calcium sodium borosilicate, benzyl benzoate, centella asiatica extract, aluminum hydroxide, citral, linalool, hexyl cinnamal, tin oxide, geraniol, eugenol, aloe barbadensis leaf extract, calcium pantothenate, honey, ascorbic acid, dimethicone, sodium chondroitin sulfate, atelocollagen; may contain: titanium dioxide, mica, red 28 lake, iron oxides, red 7, red 22 lake, yellow 5 lake, blue 1 lake, yellow 6 lake, carmine
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MAYBELLINE NEW YORK BABY LIPS MOISTURIZING LIP BALM SPF 20
octinoxate octisalate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 49967-452 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.33 g in 4.4 g Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 0.132 g in 4.4 g Inactive Ingredients Ingredient Name Strength POLYBUTENE (1400 MW) (UNII: 1NA5AO9GH7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49967-452-01 1 in 1 BLISTER PACK 08/01/2011 1 4.4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 08/01/2011 Labeler - L'Oreal USA Products Inc (002136794) Establishment Name Address ID/FEI Business Operations L'Oreal USA Products Inc 624244349 manufacture(49967-452)
Trademark Results [Maybelline New York]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MAYBELLINE NEW YORK 87141103 not registered Dead/Abandoned |
L'Oreal USA Creative, Inc. 2016-08-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.