EASOTIC OTIC- hydrocortisone aceponate, miconazole nitrate, and gentamicin sulfate suspension
Easotic by
Drug Labeling and Warnings
Easotic by is a Animal medication manufactured, distributed, or labeled by Virbac AH, Inc., Virbac Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- CAUTION
- DESCRIPTION
- INDICATIONS
-
DOSAGE AND ADMINSTRATION
Verify that the tympanic membrane is intact. Shake well before each use.
Priming the canister
Prior to the first use of the dosing canister, prime the pump by depressing the pump 1 to 2 times to fill the clear canula (tip) with a full dose of product.
Carefully insert the canula into the affected external ear canal(s) and apply 1 mL (a single pump) of suspension once per day for 5 days. Wash hands after usage.
- CONTRAINDICATIONS
-
WARNINGS
Human Warnings
Not for use in humans. Keep this and all drugs out of reach of children. In case of accidental skin contact, wash area thoroughly with water. Avoid contact with eyes.
Humans with known or suspected hypersensitivity to hydrocortisone, aminoglycoside antibiotics, or azole antifungals should not handle this product.
In case of accidental ingestion by humans, contact a physician immediately. Physicians may contact a Poison Control Center for advice concerning cases of ingestion by humans.
Animal Warnings
As a class, aminoglycoside antibiotics are associated with ototoxicity, vestibular dysfunction and renal toxicity. The use of EASOTIC® suspension in a dog with a damaged tympanic membrane can result in damage to the structures of the ear associated with hearing and balance or in transmission of the infection to the middle or inner ear. Immediately discontinue use of EASOTIC® suspension if hearing loss or signs of vestibular dysfunction are observed during treatment (see ADVERSE REACTIONS).
-
PRECAUTIONS
Do not administer orally.
Concurrent administration of potentially ototoxic drugs should be avoided.
Use with caution in dogs with impaired hepatic or renal function (see ANIMAL SAFETY).
Long-term use of topical otic corticosteroids has been associated with adrenocortical suppression and iatrogenic hyperadrenocorticism in dogs (see ANIMAL SAFETY).
The safe use of EASOTIC® suspension in dogs used for breeding purposes, during pregnancy, or in lactating bitches, has not been evaluated.
-
ADVERSE REACTIONS
In a field study conducted in the United States (see EFFECTIVENESS), there were no adverse reactions reported in 145 dogs administered EASOTIC® suspension.
In foreign market experience, reports of hearing loss and application site erythema have been received. In most reported cases, the hearing loss and erythema were transient and resolved with discontinuation of EASOTIC® suspension.
To report suspected adverse drug events, contact Virbac at 800-338-3659 or the FDA at 1-888-FDA-VETS.
For technical assistance or to obtain a Material Safety Data Sheet, call Virbac at 800-338-3659.
-
PHARMACOLOGY
Hydrocortisone aceponate is a glucocorticoid with anti-inflammatory effects. Miconazole nitrate is an imidazole antifungal. Gentamicin sulfate is an aminoglycoside antibiotic.
In the target animal safety study, hydrocortisone aceponate, miconazole and gentamicin were shown to be systemically absorbed from the ears of healthy dogs (see ANIMAL SAFETY); increased systemic absorption may be observed in inflamed ears.
-
MICROBIOLOGY
The compatibility and additive effect of each of the components in EASOTIC® suspension was demonstrated in a component effectiveness and non-interference study. An in vitro study of organisms collected from clinical cases of otitis externa in dogs and from dogs enrolled in the clinical effectiveness study for EASOTIC® suspension determined that miconazole nitrate and gentamicin sulfate inhibit the growth of bacteria and yeast commonly associated with otitis externa in dogs. No consistent synergistic or antagonistic effect of the two antimicrobials was demonstrated. The addition of hydrocortisone aceponate to the combination did not impair antimicrobial activity to any clinically-significant extent.
In a field study (see EFFECTIVENESS), the minimum of 10 isolates from successfully treated cases was met for S. pseudintermedius and M. pachydermatis.
-
EFFECTIVENESS
The effectiveness of this drug was evaluated in 157 dogs with otitis externa. The study was a double-masked field study with a placebo control. One hundred and four dogs were treated with EASOTIC® suspension and 53 dogs were treated with the placebo control. Treatment was administered once daily for 5 consecutive days to the affected ear(s). The dogs were evaluated at 4 different intervals over the course of 1 month to determine response to therapy. The 6 clinical signs evaluated were: malodor, aural discharge, pruritus, erythema, swelling and pain. The individual clinical scores were assigned based on the severity of each sign. Success was based on clinical improvement at Day 28 ±2 days. The success rates of the 2 groups were significantly different (p=0.0179); 68.5% of dogs administered EASOTIC® suspension were successfully treated, compared to 21.8% of the dogs in the placebo control group.
-
ANIMAL SAFETY
In the target animal safety study, EASOTIC® suspension was administered at 0×, 1×, 3× and 5× the recommended dose for 15 consecutive days (3 times the recommended treatment duration) in laboratory Beagles, with 8 dogs per group. Hypersensitivity reactions in the external ear canal and inner pinnae were seen in all EASOTIC® suspension groups and included mild to severe aural erythema (3× group), papules and ulceration (1× and 5× groups), otitis externa (3× and 5× groups), and otitis media (5× group). Renal tubular crystals were present in the cortex and medulla (0×, 1×, 3×, and 5× groups) and mild renal tubular basophilia and atrophy were present in one 5× group dog. Baseline cortisol values and the cortisol response to ACTH stimulation were lower in treated dogs compared to the control dogs. The ACTH stimulation test results are consistent with systemic absorption of topical corticosteroids causing suppression of the hypothalamicpituitary-adrenal axis. Dogs in the 3× and 5× groups demonstrated elevations in AST and ALP, while dogs in the 1×, 3×, and 5× groups had elevated cholesterol, total protein, and albumin levels. Dogs in the 3× and 5× groups also had higher liver weights and greater food consumption.
- STORAGE INFORMATION
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 10 mL Carton
easOtic®
(hydrocortisone aceponate 1.11 mg /
miconazole nitrate 15.1 mg /
gentamicin sulfate 1.5 mg/mL)
Suspension for DogsFor otic use in dogs only.
CAUTION:
Federal law restricts this drug
to use by or on the order of
a licensed veterinarian.Net Contents: 10 mL (10 doses)
NADA 141-330, Approved by FDA
Virbac
ANIMAL HEALTH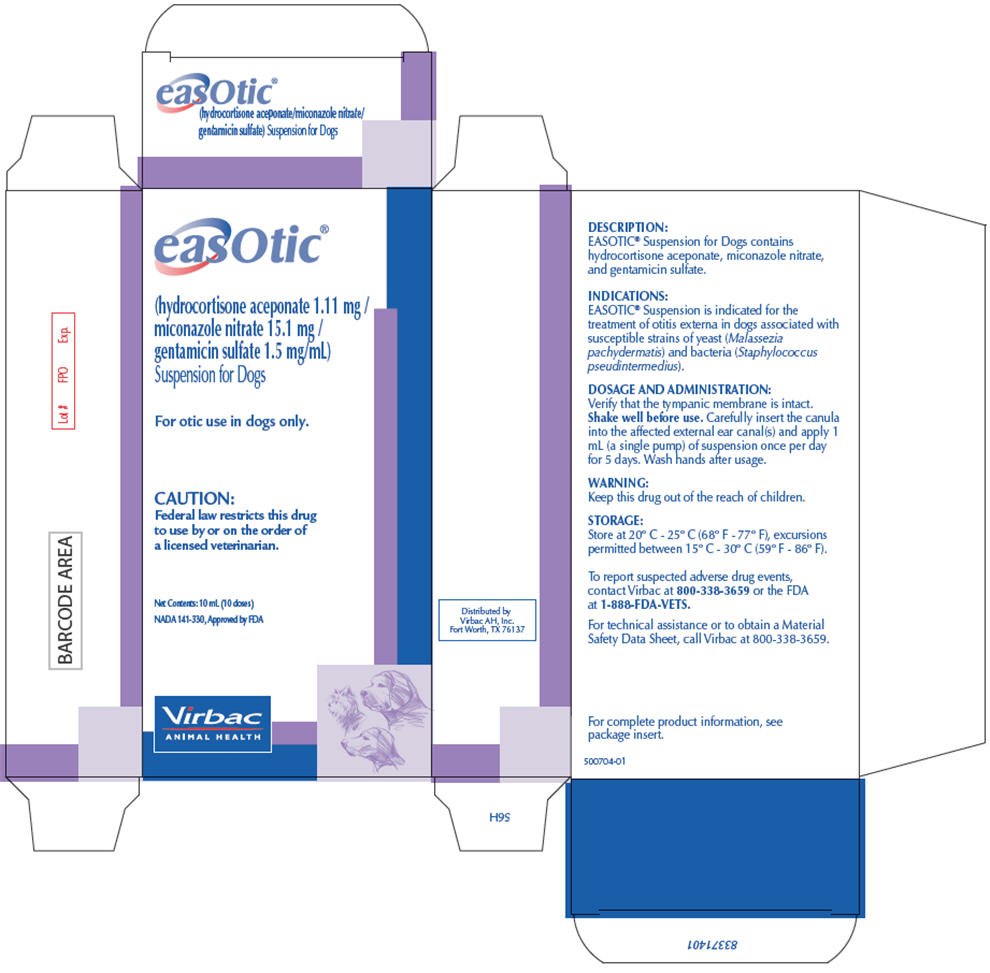
-
INGREDIENTS AND APPEARANCE
EASOTIC OTIC
hydrocortisone aceponate, miconazole nitrate, and gentamicin sulfate suspensionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 51311-360 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Hydrocortisone aceponate (UNII: 2340UP1L2G) (Hydrocortisone - UNII:WI4X0X7BPJ) Hydrocortisone aceponate 1.11 mg in 1 mL Miconazole Nitrate (UNII: VW4H1CYW1K) (Miconazole - UNII:7NNO0D7S5M) Miconazole Nitrate 15.1 mg in 1 mL Gentamicin Sulfate (UNII: 8X7386QRLV) (Gentamicin - UNII:T6Z9V48IKG) Gentamicin Sulfate 1.5 mg in 1 mL Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51311-360-10 10 mL in 1 BOTTLE, PUMP Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141330 02/07/2012 Labeler - Virbac AH, Inc. (131568396) Establishment Name Address ID/FEI Business Operations Virbac Corporation 829166276 manufacture Establishment Name Address ID/FEI Business Operations Hovione FarmaCiencia SA 449818434 api manufacture Establishment Name Address ID/FEI Business Operations FDC Limited Roha Plant 650441301 api manufacture Establishment Name Address ID/FEI Business Operations Fujian Fukang Pharmaceutical Co. LTD 528180884 api manufacture
Trademark Results [Easotic]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 EASOTIC 86809327 5179030 Live/Registered |
VIRBAC, S.A. 2015-11-04 |
 EASOTIC 79059691 3587484 Dead/Cancelled |
VIRBAC S.A. 2008-07-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.