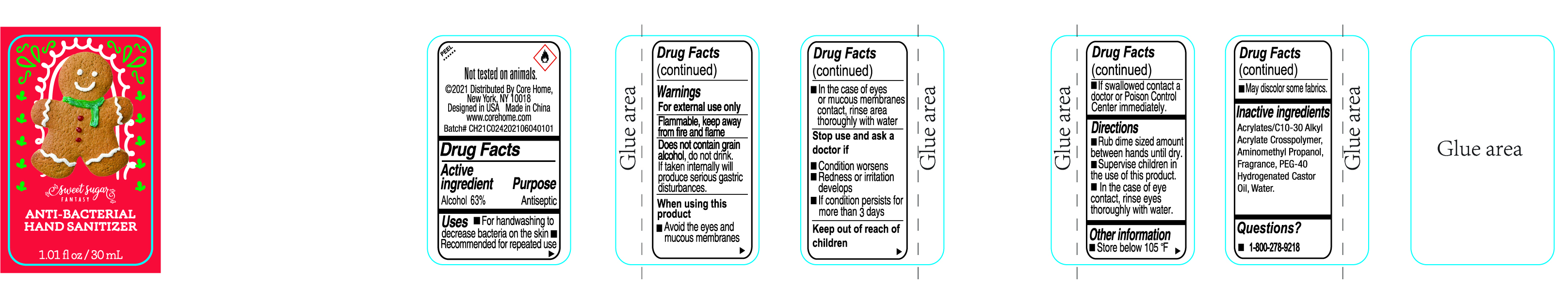
CoreFrtrssClrlHndSntzrAlch63
Sweet Sugar Fantasy Hand Sanitizer by
Drug Labeling and Warnings
Sweet Sugar Fantasy Hand Sanitizer by is a Otc medication manufactured, distributed, or labeled by Core Home. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SWEET SUGAR FANTASY HAND SANITIZER ANTIBACTERIAL- alcohol gel
Core Home
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
CoreFrtrssClrlHndSntzrAlch63
Warnings
For external use only
Flammable, keep away from fire and flame
Does not contain grain alcohol, do not drink. If taken internally will produce gastric distrubances.
When using this product
■ Avoid the eyes and mucoous membranes
■ In the case of eyes or mucous membranes contacr, rinse area thoroughly with water
Stop use and ask a doctor if
■ Condition worsens
■ Redness or irritation develops
■ Condition persists for more than 3 days
Keep out of reach of children
■ If swallowed, contact a doctor or Poison Control Center immediately.
Directions
■ Rub dime sized amount between hands until dry.
■ Supervise children in the use of this product.
■ In the case of eye contact, rinse eyes thoroughly with water.
| SWEET SUGAR FANTASY HAND SANITIZER
ANTIBACTERIAL
alcohol gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Core Home (831309427) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.