PAIN RELIEF PM EXTRA STRENGTH- acetaminophen, diphenhydramine hcl tablet, coated
Pain Relief PM by
Drug Labeling and Warnings
Pain Relief PM by is a Otc medication manufactured, distributed, or labeled by Geiss, Destin & Dunn, Inc (Goodsense), P and L Development of New York Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
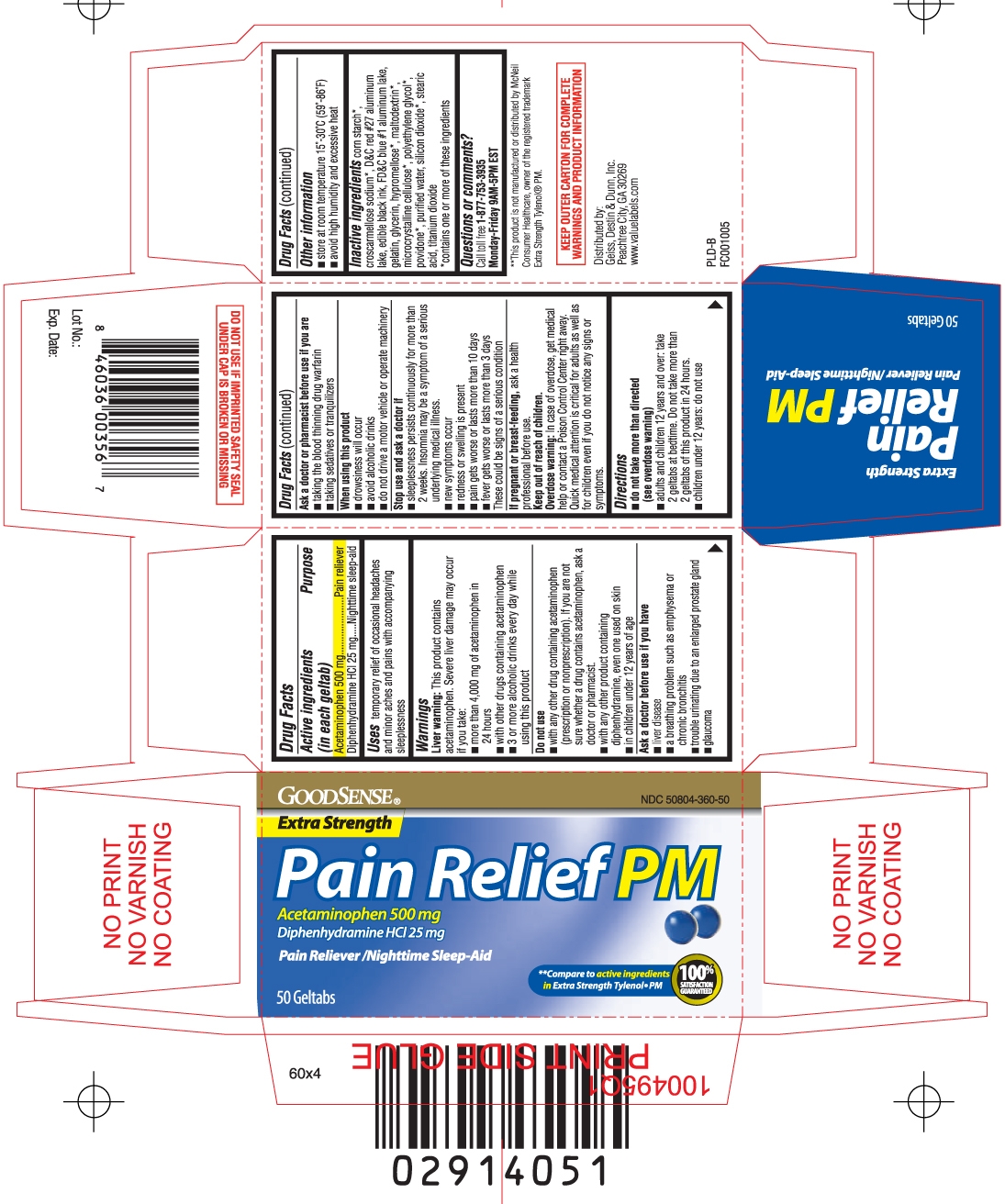
- Active ingredients (in each geltab)
- Purpose
- Uses
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- with other products containing diphenhydramine, even one used on skin
- in children under 12 years of age
Ask a doctor before use if you have
- liver disease
- a breathing problem such as emphysema or chronic bronchitis
- trouble urinating due to an enlarged prostate gland
- glaucoma
Ask a doctor or pharmacist before use if you are
- taking the blood thinning drug warfarin
- taking sedatives or tranquilizers
When using this product
- drowsiness will occur
- avoid alcoholic drinks
- do not drive a motor vehicle or operate machinery
Stop use and ask a doctor if
- sleeplessness persists continuously for more than 2 weeks. Insomnia may be a symptom of serious underlying medical illness.
- new symptoms occur
- redness or swelling is present
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
These could be signs of a serious condition
Keep out of reach of children.
Overdose warning: In case of overdose, get medical help or contact a Poison Control Center right away. Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
-
Inactive ingredients
corn starch*, croscarmellose sodium*, D&C red #27 aluminum lake, edible black ink, FD&C blue #1 aluminum lake, gelatin, glycerin, hypromellose*, maltodextrin*, microcrystalline cellulose*, polyethylene glycol*, povidone*, purified water, silicon dioxide*, stearic acid, titanium dioxide
*contains one or more of these ingredients - Questions or comments?
-
Principal Display Panel
**Compare to active ingredients in Extra Strength TYLENOL® PM
Pain Relief PM
Extra Strength
ACETAMINOPHEN 500 mg
DIPHENHYDRAMINE HCl 25 mg
- pain reliever
- nighttime sleep-aid
**This product is not manufactured or distributed by McNeil Consumer Healthcare, owner of the registered trademark Extra Strength Tylenol® PM
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION
DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
- Product Label
-
INGREDIENTS AND APPEARANCE
PAIN RELIEF PM EXTRA STRENGTH
acetaminophen, diphenhydramine hcl tablet, coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 50804-360 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 500 mg DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) HYPROMELLOSES (UNII: 3NXW29V3WO) MALTODEXTRIN (UNII: 7CVR7L4A2D) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOLS (UNII: 3WJQ0SDW1A) POVIDONES (UNII: FZ989GH94E) WATER (UNII: 059QF0KO0R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) ALUMINUM OXIDE (UNII: LMI26O6933) Product Characteristics Color BLUE, WHITE Score no score Shape ROUND Size 13mm Flavor Imprint Code BPI50 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50804-360-50 1 in 1 CARTON 1 50 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part338 10/19/2012 Labeler - Geiss, Destin & Dunn, Inc (Goodsense) (076059836) Registrant - P and L Development of New York Corporation (800014821)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.