POTASSIUM CHLORIDE IN SODIUM CHLORIDE- sodium chloride and potassium chloride injection, solution
Potassium Chloride in Sodium Chloride by
Drug Labeling and Warnings
Potassium Chloride in Sodium Chloride by is a Prescription medication manufactured, distributed, or labeled by Fresenius Kabi USA, LLC, Fresenius Kabi Norge Norway. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Potassium Chloride in Sodium Chloride Injection, USP is a sterile, nonpyrogenic, solution for fluid and electrolyte replenishment in a single dose container for intravenous administration. It contains no antimicrobial agents. Composition, osmolarity, pH and ionic concentration are shown in Table 1.
Table 1 *Normal physiologic osmolarity range is approximately 280 to 310 mOsmol/L. Administration of substantially hypertonic solutions (≥ 600 mOsmol/L) may cause vein damage.
Size (mL) Composition (g/L) *Osmolarity (mOsmol/L) (Calc.) pH Ionic Concentration (mEq/L) Sodium Chloride, USP NaCl
Potassium Chloride, USP KCl
Sodium Potassium Chloride 20 mEq/L Potassium Chloride in 0.45% Sodium Chloride Injection, USP 1000 4.5 1.5 194 5.5
(3.5 to 6.5)77 20 97 20 mEq/L Potassium Chloride in 0.9% Sodium Chloride Injection, USP 1000 9 1.5 348 5.5
(3.5 to 6.5)154 20 174 40 mEq/L Potassium Chloride in 0.9% Sodium Chloride Injection, USP 1000 9 3 388 5.5
(3.5 to 6.5)154 40 194 The flexible plastic container is fabricated from a specially formulated non-plasticized, film containing polypropylene and thermoplastic elastomers (freeflex® bag). The amount of water that can permeate from the container into the overwrap is insufficient to affect the solution significantly. Solutions in contact with the flexible container can leach out certain of the container's chemical components in very small amounts within the expiration period. The suitability of the container material has been confirmed by tests in animals according to USP biological tests for plastic containers.
- CLINICAL PHARMACOLOGY
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
Hypersensitivity
Hypersensitivity and infusion reactions, including anaphylaxis and chills, have been reported with products containing potassium chloride and sodium chloride. Stop the infusion immediately if signs or symptoms of a hypersensitivity or infusion reaction develops. Appropriate therapeutic countermeasures must be instituted as clinically indicated.
Electrolyte Imbalances
Hyperkalemia
Potassium-containing solutions, including Potassium Chloride in Sodium Chloride Injection, USP may increase the risk of hyperkalemia. Hyperkalemia can be asymptomatic and manifest only by increased serum potassium concentrations and/or characteristic electrocardiographic (ECG) changes. Cardiac conduction disorders (including complete heart block) and other cardiac arrhythmias, some fatal, can develop at any time during hyperkalemia. Continuous electrocardiogram (ECG) monitoring may be necessary to aid in the detection of cardiac arrhythmias due to hyperkalemia (see ADVERSE REACTIONS).
To avoid life threatening hyperkalemia, do not administer Potassium Chloride in Sodium Chloride Injection, USP as an intravenous push (i.e., intravenous injection manually with a syringe connected to the intravenous access) without a quantitative infusion device.
Patients at increased risk of developing hyperkalemia and cardiac arrhythmias include those:
- with conditions predisposing to hyperkalemia and/or associated with increased sensitivity to potassium, such as patients with severe renal impairment, acute dehydration, extensive tissue injury or burns, certain cardiac disorders such as congestive heart failure or atrioventricular (AV) block (especially if they receive digoxin).
- who are at risk of experiencing hyperosmolality, acidosis, or undergoing correction of alkalosis (conditions associated with a shift of potassium from intracellular to extracellular space).
- treated concurrently or recently with agents or products that can cause or increase the risk of hyperkalemia (see DRUG INTERACTIONS).
- with cardiac arrhythmias.
Avoid use of Potassium Chloride in Sodium Chloride Injection, USP in patients with, or at risk for, hyperkalemia. If use cannot be avoided, use a product with a low amount of potassium chloride, infuse slowly and monitor serum potassium concentrations and ECGs.
Hypernatremia and Hyperchloremia
Electrolyte imbalances such as hypernatremia, hyperchloremia, and metabolic acidosis may occur with Potassium Chloride in Sodium Chloride Injection, USP.
Conditions that may increase the risk of hypernatremia, fluid overload and edema (central and peripheral), include patients with: primary hyperaldosteronism; secondary hyperaldosteronism associated with, for example, hypertension, congestive heart failure, liver disease (including cirrhosis), renal disease (including renal artery stenosis, nephrosclerosis); and pre-eclampsia.
Certain medications, such as corticosteroids or corticotropin, may also increase risk of sodium and fluid retention, see DRUG INTERACTIONS.
Avoid Potassium Chloride in Sodium Chloride Injection, USP in patients with, or at risk for, hypernatremia or hyperchloremia. If use cannot be avoided, monitor serum sodium and chloride concentrations and acid-base balance.
Rapid correction of hypernatremia is potentially dangerous with risk of serious neurologic complications. Excessively rapid correction of hypernatremia is also associated with a risk for serious neurologic complications such as osmotic demyelination syndrome (ODS) with risk of seizures and cerebral edema.
Hyponatremia
Potassium Chloride in Sodium Chloride Injection, USP may cause hyponatremia. Hyponatremia can lead to acute hyponatremic encephalopathy characterized by headache, nausea, seizures, lethargy, and vomiting. Patients with brain edema are at particular risk of severe, irreversible and life-threatening brain injury.
The risk of hospital-acquired hyponatremia is increased in patients with cardiac or pulmonary failure, and in patients with non-osmotic vasopressin release (including SIADH) treated with high volume of hypotonic Potassium Chloride in Sodium Chloride Injection, USP.
The risk for hyponatremia is increased in pediatric patients, elderly patients, postoperative patients, those with psychogenic polydipsia, and in patients treated with medications that increase the risk of hyponatremia (such as diuretics, certain antiepileptic and psychotropic medications). See DRUG INTERACTIONS.
Patients at increased risk for developing complications of hyponatremia such as hyponatremic encephalopathy, include pediatric patients, women (in particular, premenopausal women), patients with hypoxemia, and patients with underlying central nervous system disease. Avoid Potassium Chloride in Sodium Chloride Injection, USP in patients with or at risk for hyponatremia. If use cannot be avoided, monitor serum sodium concentrations.
Rapid correction of hyponatremia is potentially dangerous with risk of serious neurologic complications. Brain adaptations reducing risk of cerebral edema make the brain vulnerable to injury when chronic hyponatremia is too rapidly corrected, which is known as osmotic demyelination syndrome (ODS). To avoid complications, monitor serum sodium and chloride concentrations, fluid status, acid-base balance, and signs of neurologic complications.
Fluid Overload
Depending on the volume and rate of infusion, and the patient’s underlying clinical condition, the intravenous administration of Potassium Chloride in Sodium Chloride Injection, USP can cause electrolyte disturbances such as overhydration/hypervolemia and congested states including central (e.g., pulmonary edema) and peripheral edema.
Avoid Potassium Chloride in Sodium Chloride Injection, USP in patients with or at risk for fluid and/or solute overloading. If use cannot be avoided, monitor fluid balance, electrolyte concentrations and acid base balance as needed and especially during prolonged use.
-
PRECAUTIONS
Patients with Severe Renal Impairment
Administration of sodium and potassium in patients with or at risk of severe renal impairment, may result in hypernatremia, hyperkalemia and/or fluid overload (see WARNINGS). Avoid Potassium Chloride in Sodium Chloride Injection, USP in patients with severe renal impairment. If use cannot be avoided, monitor patients with severe renal impairment for development of these adverse reactions.
Drug Interactions
Lithium
Renal sodium and lithium clearance may be increased during administration of Potassium Chloride in Sodium Chloride Injection, USP and result in decreased lithium concentrations. Monitor serum lithium concentrations during concomitant use.
Other Products that Cause Hyperkalemia
Administration of Potassium Chloride in Sodium Chloride Injection, USP in patients treated concurrently or recently with products that are associated with hyperkalemia increases the risk of severe and potentially fatal hyperkalemia, in particular in the presence of other risk factors for hyperkalemia. Avoid use of Potassium Chloride in Sodium Chloride Injection, USP in patients receiving such products (e.g., potassium sparing diuretics, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or the immunosuppressants cyclosporine and tacrolimus). If use cannot be avoided, monitor serum potassium concentrations.
Other Products that Affect Fluid and/or Electrolyte Balance
Administration of Potassium Chloride in Sodium Chloride Injection, USP in patients treated concomitantly with medications associated with sodium and fluid retention may increase the risk of hypernatremia and volume overload. Avoid use of Potassium Chloride in Sodium Chloride Injection, USP in patients receiving such products, such as corticosteroids or corticotropin. If use cannot be avoided, monitor serum electrolytes, fluid balance, and acid-base balance.
Other Drugs that Increase the Risk of Hyponatremia
Administration of Potassium Chloride in Sodium Chloride Injection, USP in patients treated concomitantly with medications associated with hyponatremia may increase the risk of developing hyponatremia.
Avoid use of Potassium Chloride in Sodium Chloride Injection, USP in patients receiving products, such as diuretics, and certain antiepileptic and psychotropic medications. Drugs that increase the vasopressin effect reduce renal electrolyte free water excretion and may also increase the risk of hyponatremia following treatment with intravenous fluids. If use cannot be avoided, monitor serum sodium concentrations.
Pregnancy
There are no adequate and well controlled studies from the use of Potassium Chloride in Sodium Chloride Injection, USP in pregnant or lactating women and animal reproduction studies have not been conducted with this drug. Therefore, it is also not known whether Potassium Chloride in Sodium Chloride Injection, USP can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Potassium Chloride in Sodium Chloride Injection, USP should be given to a pregnant woman only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Potassium Chloride in Sodium Chloride Injection, USP is administered to a nursing mother.
Pediatric Use
The use of Potassium Chloride in Sodium Chloride Injection, USP in pediatric patients is based on clinical practice. (See DOSAGE AND ADMINISTRATION). Safety and effectiveness of Potassium Chloride in Sodium Chloride Injection, USP in pediatric patients have not been established by adequate and well-controlled studies.
Pediatric patients are at increased risk of developing hyponatremia as well as for developing encephalopathy as a complication of hyponatremia (see WARNINGS).
Geriatric Use
Geriatric patients are at increased risk of developing electrolyte imbalances. Potassium Chloride in Sodium Chloride Injection, USP is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function.
Therefore, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. Consider monitoring renal function in elderly patients.
-
ADVERSE REACTIONS
The following adverse reactions associated with the use of Potassium Chloride in Sodium Chloride Injection, USP were identified in clinical trials or postmarketing reports. Because postmarketing reactions were reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency, reliably, or to establish a causal relationship to drug exposure.
General disorders and administration site conditions: Chills, and infusion site pain.
Hypersensitivity reactions: generalized papules and erythema, rash, fever, vomiting, hypertension, tachycardia.
Metabolism and nutrition disorders: Hyperkalemia, hyponatremia, hypernatremia, hyperchloremia acidosis, fluid overload.
Cardiac disorders: Cardiac arrest as a manifestation of rapid intravenous administration and/or of hyperkalemia.
Nervous System Disorders: Hyponatremic encephalopathy.
If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate therapeutic countermeasures and save the remainder of the fluid for examination if deemed necessary.
-
OVERDOSAGE
An increased infusion rate of Potassium Chloride in Sodium Chloride Injection, USP can cause:
- hyperkalemia, manifestations may include disturbances in cardiac conduction and arrhythmias, including bradycardia, heart block, asystole, ventricular tachycardia, ventricular fibrillation.
The presence of any ECG findings that are suspected to be caused by hyperkalemia should be considered a medical emergency.
If hyperkalemia is present or suspected, discontinue the infusion immediately and institute close ECG, laboratory and other monitoring and, as necessary, corrective therapy to reduce serum potassium concentrations.
Muscle weakness (up to and including muscular and respiratory paralysis, paresthesia of extremities) may occur as a complication of hyperkalemia. - hyponatremia, manifestations may include seizures, coma, cerebral edema and death.
- hypernatremia, especially in patients with severe renal impairment.
- hypotension.
- gastrointestinal symptoms (ileus, nausea, vomiting, abdominal pain).
- fluid overload (which can lead to central and/or peripheral edema). See WARNINGS and ADVERSE REACTIONS.
When assessing an overdose, any additives in the solution must also be considered. The effects of an overdose may require immediate medical attention and treatment. Interventions include discontinuation of Potassium Chloride in Sodium Chloride Injection, USP administration, dose reduction, and other measures as indicated for the specific clinical constellation (e.g., monitoring of fluid balance, electrolyte concentrations and acid-base balance).
- hyperkalemia, manifestations may include disturbances in cardiac conduction and arrhythmias, including bradycardia, heart block, asystole, ventricular tachycardia, ventricular fibrillation.
-
DOSAGE AND ADMINISTRATION
Important Administration Instructions
- Potassium Chloride in Sodium Chloride Injection, USP is intended for intravenous infusion using sterile equipment.
- To avoid life threatening hyperkalemia, do not administer Potassium Chloride in Sodium Chloride Injection, USP as an intravenous push (i.e., intravenous injection manually with a syringe connected to the intravenous access) without a quantitative infusion device (see WARNINGS).
- Do not connect flexible plastic containers in series in order to avoid air embolism due to possible residual air contained in the primary container.
- Set the vent to the closed position on a vented intravenous administration set to prevent air embolism.
- Use a dedicated line without any connections to avoid air embolism.
- Do not pressurize intravenous solutions contained in flexible plastic containers to increase flow rates in order to avoid air embolism due to incomplete evacuation of residual air in the container.
- The choice of a central or peripheral venous route of infusion should depend on the osmolarity of the final infusate. Solutions with osmolarity of greater than or equal to approximately 900 mOsm/L must be infused through a central catheter.
- Prior to infusion, visually inspect the solution for particulate matter and discoloration. The solution should be clear and there should be no precipitates. Do not administer unless solution is clear and container is undamaged.
- Use of final filter is recommended during administration of all parenteral solutions, where possible.
Dosing Information
The choice of the specific potassium chloride and sodium chloride formulation, dosage, volume, rate and duration of administration is dependent upon the age, weight and clinical and metabolic condition of the patient and concomitant therapy, and administration should be determined by a physician experienced in intravenous fluid therapy.
Additional electrolyte supplementation may be indicated according to the clinical needs of the patient. Additives can be introduced to the container; however, some additives may be incompatible. Evaluate all additions to the plastic container for compatibility and stability of the resulting preparation. Consult with a pharmacist, if available.
If, in the informed judgment of the physician, it is deemed advisable to introduce additives, use aseptic technique. After addition, if there is a discoloration and/or the appearance of precipitates, insoluble complexes or crystals, do not use. Mix thoroughly when additives have been introduced. Do not store solutions containing additives. Discard any unused portion.
Rapid correction of hyponatremia and hypernatremia is potentially dangerous (risk of serious neurologic complications). To avoid complications such as osmotic demyelination syndrome (ODS) during administration, follow the important administration instructions, monitor serum sodium and chloride concentrations, fluid status, acid-base balance, and signs of neurologic complications.
-
HOW SUPPLIED
Potassium Chloride in Sodium Chloride Injection, USP is supplied in single-dose flexible plastic containers as follows:
Product Code Unit of Use Strength Unit of Sale 683110 NDC: 63323-683-01
One 1000 mL freeflex® bag20 mEq Potassium Chloride in 0.45% Sodium Chloride NDC: 63323-683-10
Package of 10 freeflex® bags686110 NDC: 63323-686-01
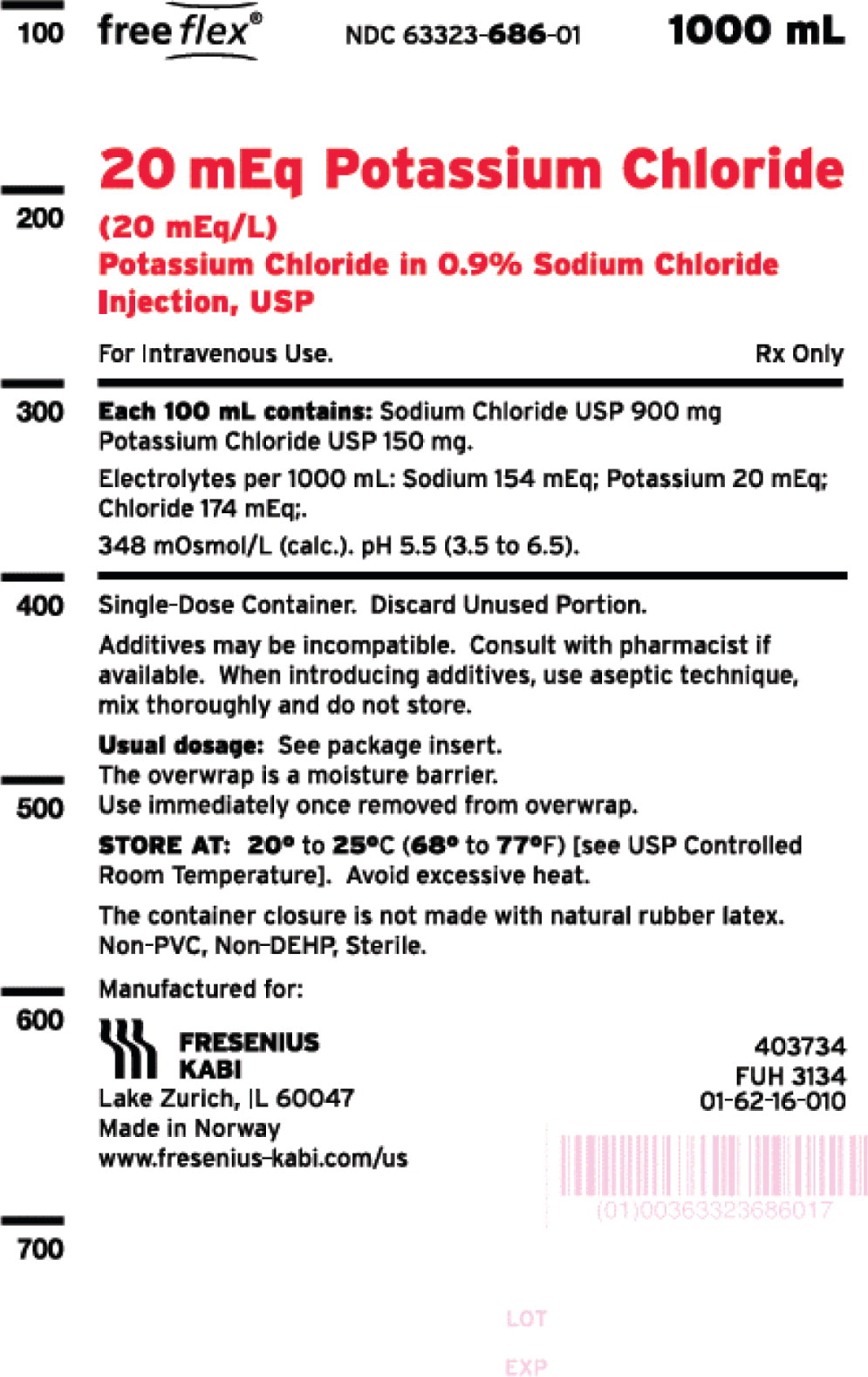
One 1000 mL freeflex® bag20 mEq Potassium Chloride in 0.9% Sodium Chloride NDC: 63323-686-10
Package of 10 freeflex® bags688110 NDC: 63323-688-01
One 1000 mL freeflex® bag40 mEq Potassium Chloride in 0.9% Sodium Chloride NDC: 63323-688-10
Package of 10 freeflex® bagsExposure of pharmaceutical products to heat should be minimized. Avoid excessive heat. It is recommended the product be stored between 20ºC to 25°C (68º F to 77°F). [See USP controlled room temperature.]; brief exposure up to 40°C (104°F) does not adversely affect the product.
The container closure is not made with natural rubber latex. Non-PVC, Non-DEHP, Sterile.
-
INSTRUCTIONS FOR USE:
Check flexible container solution composition, lot number, and expiry date.
Do not remove solution container from its overwrap until immediately before use. Use sterile equipment and aseptic technique.
Flexible Plastic Container (freeflex® bag)
To Open
- Turn solution container over so that the text is face down. Using the pre-cut corner tabs, peel open the overwrap and remove solution container.
- Check the solution container for leaks by squeezing firmly. If leaks are found, or if the seal is not intact, discard the solution.
- Do not use if the solution is cloudy or a precipitate is present.
Preparation for Administration
- Immediately before inserting the infusion set, break off BLUE Infusion Port Cap with the arrow pointing away from container.
- Use a non-vented infusion set or close the air-inlet on a vented set.
- Close the roller clamp of the infusion set.
- Hold the base of BLUE Infusion Port.
- Insert spike through BLUE Infusion Port by rotating wrist slightly until the spike is inserted. NOTE: See full directions accompanying administration set.
To Add Medication Prior to Solution Administration
- Identify WHITE Additive Port with arrow pointing toward container.
- Immediately before injecting additives, break off WHITE Additive Port Cap with the arrow pointing toward container.
- Hold base of WHITE Additive Port horizontally.
- Prepare medication site
- Insert an 18 to 23 gauge needle horizontally through the center of WHITE Additive Port's septum and inject additives.
- Mix container contents thoroughly.
For high density medication such as potassium chloride, squeeze ports while ports are upright and mix thoroughly.
To Add Medication During Solution Administration
- Close the clamp on the set
- Identify WHITE Additive Port with arrow pointing toward container
- Immediately before injecting additives, if the Cap has not been broken off, break off WHITE Additive Port cap with the arrow pointing toward container.
- Hold base of WHITE Additive Port horizontally.
- Prepare medication site.
- Using a syringe with an 18 to 23 gauge needle, horizontally insert through the center of WHITE Additive Port's septum and inject additives.
- Remove container from IV pole and/or turn to an upright position.
- Mix container contents thoroughly.
- Using aseptic technique, repeat steps 4-7 as necessary.
- Return container to in use position and continue administration.
WARNING: Do not use flexible container in series connections.
Manufactured for:
Lake Zurich, IL 60047
Made in Norwaywww.fresenius-kabi.com/us
451701
Issued: February 2021 - PACKAGE LABEL - PRINCIPAL DISPLAY – Potassium Chloride in 0.45% Sodium Chloride Injection, USP Bag Label
- PACKAGE LABEL - PRINCIPAL DISPLAY – Potassium Chloride in 0.9% Sodium Chloride Injection, USP Bag Label
- PACKAGE LABEL - PRINCIPAL DISPLAY – Potassium Chloride in 0.9% Sodium Chloride Injection, USP Bag Label
-
INGREDIENTS AND APPEARANCE
POTASSIUM CHLORIDE IN SODIUM CHLORIDE
sodium chloride and potassium chloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 63323-683 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sodium Chloride (UNII: 451W47IQ8X) (Sodium Cation - UNII:LYR4M0NH37, Chloride Ion - UNII:Q32ZN48698) Sodium Chloride 450 mg in 100 mL Potassium Chloride (UNII: 660YQ98I10) (Potassium Cation - UNII:295O53K152, Chloride Ion - UNII:Q32ZN48698) Potassium Chloride 150 mg in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63323-683-10 10 in 1 CARTON 06/02/2021 1 NDC: 63323-683-01 1000 mL in 1 BAG; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA212347 06/02/2021 POTASSIUM CHLORIDE IN SODIUM CHLORIDE
sodium chloride and potassium chloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 63323-686 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sodium Chloride (UNII: 451W47IQ8X) (Sodium Cation - UNII:LYR4M0NH37, Chloride Ion - UNII:Q32ZN48698) Sodium Chloride 900 mg in 100 mL Potassium Chloride (UNII: 660YQ98I10) (Potassium Cation - UNII:295O53K152, Chloride Ion - UNII:Q32ZN48698) Potassium Chloride 150 mg in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63323-686-10 10 in 1 CARTON 06/02/2021 1 NDC: 63323-686-01 1000 mL in 1 BAG; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA212347 06/02/2021 POTASSIUM CHLORIDE IN SODIUM CHLORIDE
sodium chloride and potassium chloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 63323-688 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sodium Chloride (UNII: 451W47IQ8X) (Sodium Cation - UNII:LYR4M0NH37, Chloride Ion - UNII:Q32ZN48698) Sodium Chloride 900 mg in 100 mL Potassium Chloride (UNII: 660YQ98I10) (Potassium Cation - UNII:295O53K152, Chloride Ion - UNII:Q32ZN48698) Potassium Chloride 300 mg in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63323-688-10 10 in 1 CARTON 06/02/2021 1 NDC: 63323-688-01 1000 mL in 1 BAG; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA212347 06/02/2021 Labeler - Fresenius Kabi USA, LLC (608775388) Establishment Name Address ID/FEI Business Operations HP Halden Pharma AS 347747373 ANALYSIS(63323-683, 63323-686, 63323-688) , MANUFACTURE(63323-683, 63323-686, 63323-688) , PACK(63323-683, 63323-686, 63323-688)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.