Hand Sanitizer Wipes (BZK) - Soft Wet Wipes
Soft Wet Wipes by
Drug Labeling and Warnings
Soft Wet Wipes by is a Otc medication manufactured, distributed, or labeled by Rite-Kem Incorporated. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SOFT WET WIPES- soft wet wipes liquid
Rite-Kem Incorporated
----------
Hand Sanitizer Wipes (BZK) - Soft Wet Wipes
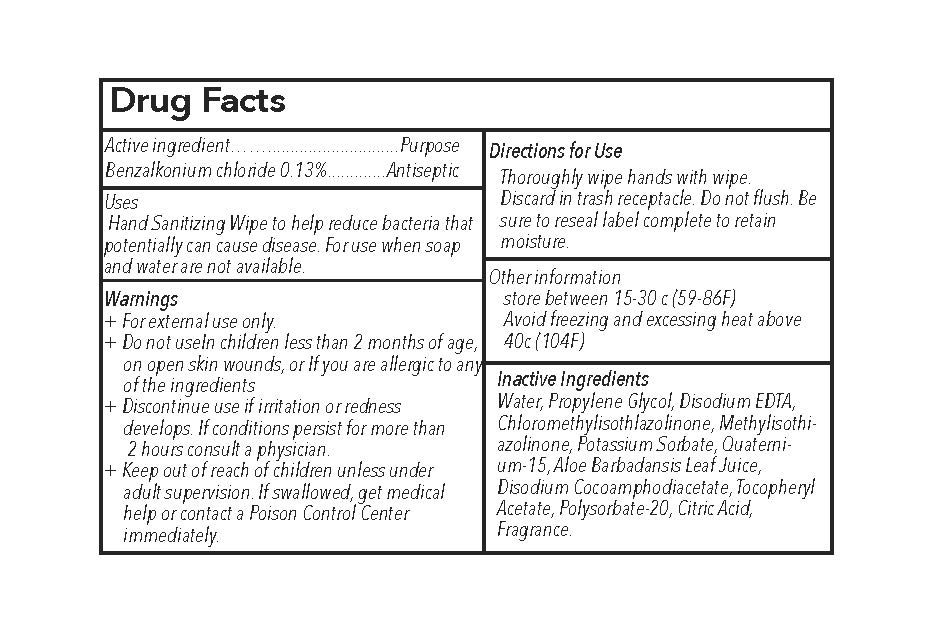
Hand Sanitizing Wipe to help reduce bacteria that potentially can cause disease. For use when soap and water are not available.
- In children less than 2 months of age
- on open skin wounds
- If you are allergic to any of the ingredients
Discontinue use if irritiation or redness develops. If conditions persist for more than 2 hours consult a physician.
Keep out of reach of children unless under adult supervision. If swallowed, get medical help or contact a poison control center immediately.
Directions for Use:
- Thoroughly wipe hands with wipe.
- Discard in trash receptacle.
- Do not flush.
- Be sure to reseal label completely to retain moisture.
Other information
store between 15-30 c (59-86F)
Avoid freezing and excessing heat above 40 c (104F)
Inactive ingredients: Water, Propylene Glycol, Disodium EDTA, Chloromethylisothlazolinone, Methylisothiazolinone, Potassium Sorbate, Quaternium-15, Aloe Barbadansis Leaf Juice, Disodium Cocoamphodiacetate, Tocopheryl Acetate, Polysorbate-20, Citric Acid, Fragrance.
Soft Wet Wipes
(15 Count)
See Back Panel for Use Information
RITE-KEM
Sold by Rite-Kem Inc. T
703 Westmoreland Tupelo MS 38801
www.RITE-KEM.com
NDC: 73943-950-01

| SOFT WET WIPES
soft wet wipes liquid |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Rite-Kem Incorporated (786892927) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Rite-Kem Incorporated | 786892927 | manufacture(73943-950) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.