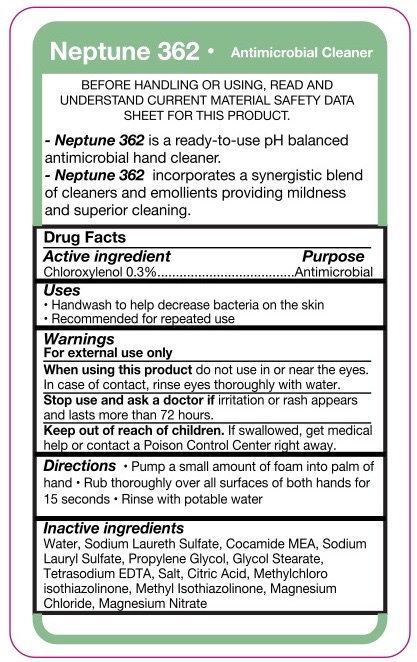
Neptune 362 Amtibacterial Hand Soap
Neptune 362 Antibacterial by
Drug Labeling and Warnings
Neptune 362 Antibacterial by is a Otc medication manufactured, distributed, or labeled by R.L. Williams Company. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
NEPTUNE 362 ANTIBACTERIAL- chloroxyenol soap
R.L. Williams Company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Neptune 362 Amtibacterial Hand Soap
Warnings
- For external use only
Directions
- Pump a small amount of foam into palm of hand.
- Rub thoroughly over all surfaces of both hands for 15 seconds.
- Rinse with potable water.
| NEPTUNE 362 ANTIBACTERIAL
chloroxyenol soap |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - R.L. Williams Company (099976362) |
Revised: 7/2022
Document Id: e354f89e-6ba8-6da1-e053-2995a90a0fe4
Set id: c24f3583-2b25-dc79-e053-2995a90a5847
Version: 2
Effective Time: 20220708
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.