GIAPREZA- angiotensin ii injection
Giapreza by
Drug Labeling and Warnings
Giapreza by is a Prescription medication manufactured, distributed, or labeled by La Jolla Pharmaceutical Company, Cangene Biopharma, LLC dba Emergent BioSolutions. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use GIAPREZA™ safely and effectively. See full prescribing information for GIAPREZA.
GIAPREZA (angiotensin II) Injection for Intravenous Infusion
Initial U.S. Approval: 2017INDICATIONS AND USAGE
GIAPREZA is a vasoconstrictor to increase blood pressure in adults with septic or other distributive shock. ( 1)
DOSAGE AND ADMINISTRATION
Dilute GIAPREZA in 0.9% sodium chloride prior to use. See Full Prescribing Information for instructions on preparation and administration of injection. Diluted solution may be stored at room temperature or under refrigeration and should be discarded after 24 hours. GIAPREZA must be administered as an intravenous infusion. ( 2.1)
- Start GIAPREZA intravenously at 20 nanograms (ng)/kg/min. Titrate as frequently as every 5 minutes by increments of up to 15 ng/kg/min as needed. During the first 3 hours, the maximum dose should not exceed 80 ng/kg/min. Maintenance dose should not exceed 40 ng/kg/min. ( 2.2)
DOSAGE FORMS AND STRENGTHS
Injection: 2.5 mg/mL and 5 mg/2 mL (2.5 mg/mL) in a vial. (3)
CONTRAINDICATIONS
None (4.1) (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
The most common adverse reactions reported in greater than 10% in GIAPREZA treated patients were thromboembolic events. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact La Jolla Pharmaceutical Company at 1-800-651-3861 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Revised: 1/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1. INDICATIONS AND USAGE
2. DOSAGE AND ADMINISTRATION
2.1. Preparation
2.2. Administration
3. DOSAGE FORMS AND STRENGTHS
4. CONTRAINDICATIONS
5. WARNINGS AND PRECAUTIONS
5.1 Risk for Thrombosis
6. ADVERSE REACTIONS
6.1. Clinical Trials Experience
7. DRUG INTERACTIONS
7.1. Angiotensin Converting Enzyme (ACE) Inhibitors
7.2. Angiotensin II Receptor Blockers (ARB)
8. USE IN SPECIFIC POPULATIONS
8.1. Pregnancy
8.2. Lactation
8.4. Pediatric Use
8.5. Geriatric Use
10. OVERDOSAGE
11. DESCRIPTION
12. CLINICAL PHARMACOLOGY
12.1. Mechanism of Action
12.2. Pharmacodynamics
12.3. Pharmacokinetics
13. NONCLINICAL TOXICOLOGY
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2. Animal Toxicology and/or Pharmacology
13.3. Safety Pharmacology
14. CLINICAL STUDIES
14.1. ATHOS-3
16. HOW SUPPLIED/STORAGE AND HANDLING
16.1. How Supplied
16.2. Storage and Handling
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1. INDICATIONS AND USAGE
-
2. DOSAGE AND ADMINISTRATION
2.1. Preparation
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
GIAPREZA must be administered as an intravenous infusion. GIAPREZA must be diluted in 0.9% sodium chloride prior to use. Dilute the contents of one vial of GIAPREZA in 0.9% saline to achieve a final concentration of 5,000 ng/mL or 10,000 ng/mL.
Discard vial and any unused portion of the drug product after use.
Table 1: Preparation of Diluted Solution Fluid Restricted? Vial Strength Withdraw Amount (mL) Infusion Bag Size (mL) Final Concentration (ng/mL) No 2.5 mg/mL 1 500 5,000 Yes 2.5 mg/mL 1 250 10,000 5 mg/2 mL 2 500 10,000 Diluted solution may be stored at room temperature or under refrigeration. Discard prepared solution after 24 hours at room temperature or under refrigeration.
2.2. Administration
The recommended starting dosage of GIAPREZA is 20 nanograms (ng)/kg/min via continuous intravenous infusion. Administration through a central venous line is recommended.
Monitor blood pressure response and titrate GIAPREZA every 5 minutes by increments of up to 15 ng/kg/min as needed to achieve or maintain target blood pressure. Do not exceed 80 ng/kg/min during the first 3 hours of treatment. Maintenance doses should not exceed 40 ng/kg/min. Doses as low as 1.25 ng/kg/min may be used.
Once the underlying shock has sufficiently improved, down-titrate every 5 to 15 minutes by increments of up to 15 ng/kg/min based on blood pressure.
- 3. DOSAGE FORMS AND STRENGTHS
- 4. CONTRAINDICATIONS
-
5. WARNINGS AND PRECAUTIONS
5.1 Risk for Thrombosis
The safety of GIAPREZA was evaluated in 321 adults with septic or other distributive shock in a randomized, double-blind, placebo-controlled study, ATHOS-3. There was a higher incidence of arterial and venous thrombotic and thromboembolic events in patients who received GIAPREZA compared to placebo-treated patients in the ATHOS-3 study (13% vs. 5%). The major imbalance was in deep venous thromboses. Use concurrent venous thromboembolism (VTE) prophylaxis.
-
6. ADVERSE REACTIONS
6.1. Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
ATHOS-3
The safety of GIAPREZA was evaluated in ATHOS-3 [see Warnings and Precautions(5.1)] . Patients in ATHOS-3 were receiving other vasopressors in addition to GIAPREZA or placebo, which were titrated to effect on mean arterial pressure (MAP).
Table 2 summarizes adverse reactions with an incidence of at least 4% among patients treated with GIAPREZA and with a rate of at least 1.5% higher with GIAPREZA than with placebo.
Table 2: Adverse Reactions Occurring in ≥ 4% of Patients Treated with GIAPREZA and ≥ 1.5% More Often than in Placebo-treated Patients in ATHOS-3 Adverse Event GIAPREZA
N=163Placebo
N=158- * Including arterial and venous thrombotic events
Thromboembolic events * 21 (12.9%) 8 (5.1%) Deep vein thrombosis 7 (4.3%) 0 (0.0%) Thrombocytopenia 16 (9.8%) 11 (7.0%) Tachycardia 14 (8.6%) 9 (5.7%) Fungal infection 10 (6.1%) 2 (1.3%) Delirium 9 (5.5%) 1 (0.6%) Acidosis 9 (5.5%) 1 (0.6%) Hyperglycemia 7 (4.3%) 4 (2.5%) Peripheral ischemia 7 (4.3%) 4 (2.5%) - 7. DRUG INTERACTIONS
-
8. USE IN SPECIFIC POPULATIONS
8.1. Pregnancy
Risk Summary
The published data on angiotensin II use in pregnant women are not sufficient to determine a drug-associated risk of adverse developmental outcomes. Animal reproduction studies have not been conducted with GIAPREZA.
All pregnancies have a background risk of birth defects, loss, or other adverse outcomes. The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Septic or other distributive shock is a medical emergency that can be fatal if left untreated. Delaying treatment in pregnant women with hypotension associated with septic or other distributive shock is likely to increase the risk of maternal and fetal morbidity and mortality.
- 10. OVERDOSAGE
-
11. DESCRIPTION
Angiotensin II is a naturally occurring peptide hormone of the renin-angiotensin-aldosterone system (RAAS) that causes vasoconstriction and an increase in blood pressure. GIAPREZA is a sterile, aqueous solution of synthetic human angiotensin II for intravenous administration by infusion. Each 1 mL of GIAPREZA contains 2.5 mg angiotensin II equivalent to an average of 2.9 mg angiotensin II acetate, 25 mg mannitol, and Water for Injection adjusted with sodium hydroxide and/or hydrochloric acid to pH of 5.5.
The chemical name of the synthetic angiotensin II acetate is L-Aspartyl-L-arginyl-L-valyl-Ltyrosyl-L-isoleucyl-L-histidyl-L-prolyl-L-phenylalanine, acetate salt. The counter ion acetate is present in a non-stoichiometric ratio. It is a white to off-white powder, soluble in water.
The structure of angiotensin II acetate is shown below.
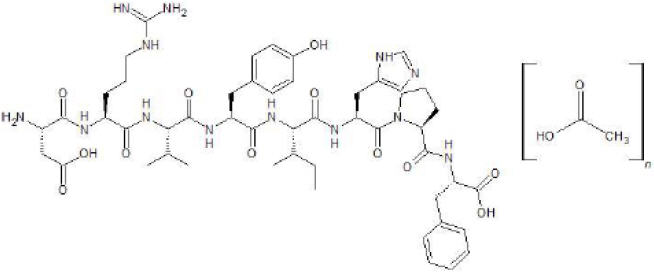
Molecular formula: C 50H 71N 13O 12 ∙ (C 2H 4O 2) n; (n= number of acetate molecules; theoretical n = 3)
Average molecular weight: 1046.2 (as free base).
-
12. CLINICAL PHARMACOLOGY
12.1. Mechanism of Action
Angiotensin II raises blood pressure by vasoconstriction and increased aldosterone release. Direct action of angiotensin II on the vessel wall is mediated by binding to the G-protein-coupled angiotensin II receptor type 1 on vascular smooth muscle cells, which stimulates Ca 2+/calmodulin-dependent phosphorylation of myosin and causes smooth muscle contraction.
12.2. Pharmacodynamics
For the 114 (70%) patients in the GIAPREZA arm who reached the target MAP at Hour 3, the median time to reach the target MAP endpoint was approximately 5 minutes. GIAPREZA is titrated to effect for each individual patient.
12.3. Pharmacokinetics
Following intravenous infusion of angiotensin II in adults with septic or other distributive shock, serum levels of angiotensin II are similar at Baseline and Hour 3 after intravenous infusion. After 3 hours of treatment, however, the serum level of angiotensin I (the angiotensin II precursor peptide) is reduced by approximately 40%.
Metabolism and Excretion:
No specific studies were conducted that examined the metabolism and excretion of GIAPREZA.
The plasma half-life of IV administered angiotensin II is less than one minute. It is metabolized by aminopeptidase A and angiotensin converting enzyme 2 to angiotensin-(2-8) [angiotensin III] and angiotensin-(1-7), respectively in plasma, erythrocytes and many of the major organs (i.e., intestine, kidney, liver and lung). Angiotensin II type 1 receptor (AT1) mediated activity of angiotensin III is approximately 40% of angiotensin II; however, aldosterone synthesis activity is similar to angiotensin II. Angiotensin-(1-7) exerts the opposite effects of angiotensin II on AT1 receptors and causes vasodilation.
Specific Populations
No formal pharmacokinetic studies were conducted with GIAPREZA in the following specific populations.
Renal Impairment
The clearance of angiotensin II is not dependent on renal function. Therefore, the pharmacokinetics of GIAPREZA are not expected to be influenced by renal impairment.
Hepatic Impairment
The clearance of angiotensin II is not dependent on hepatic function. Therefore, the pharmacokinetics of GIAPREZA are not expected to be influenced by hepatic impairment.
-
13. NONCLINICAL TOXICOLOGY
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
No genetic toxicity studies have been conducted with GIAPREZA. No carcinogenicity or fertility studies with GIAPREZA have been conducted in animals.
13.2. Animal Toxicology and/or Pharmacology
No animal toxicology studies were conducted with GIAPREZA.
13.3. Safety Pharmacology
In a cardiovascular safety pharmacology study in normotensive dogs, GIAPREZA doses of 150, 450, and 1800 ng/kg (5, 15 and 60 ng/kg/min) were infused intravenously for 30 minutes each. At ≥ 450 ng/kg, GIAPREZA caused significantly elevated MAP and systemic vascular resistance, as expected. The 1800 ng/kg dose also caused increased heart rate, increased systemic vascular resistance, increased left ventricular systolic and end-diastolic pressures, and PR interval prolongation. GIAPREZA did not significantly alter respiratory rate or cause electrocardiographic changes in QRS duration or QTc.
-
14. CLINICAL STUDIES
14.1. ATHOS-3
The Angiotensin II for the Treatment of High-Output Shock (ATHOS-3) trial was a double-blind study in which 321 adults with septic or other distributive shock who remained hypotensive despite fluid and vasopressor therapy were randomized 1:1 to GIAPREZA or placebo. Doses of GIAPREZA or placebo were titrated to a target mean arterial pressure (MAP) of ≥ 75 mmHg during the first 3 hours of treatment while doses of other vasopressors were maintained. From Hour 3 to Hour 48, GIAPREZA or placebo were titrated to maintain MAP between 65 and 70 mmHg while reducing doses of other vasopressors. The primary endpoint was the percentage of subjects who achieved either a MAP ≥ 75 mmHg or a ≥ 10 mmHg increase in MAP without an increase in baseline vasopressor therapy at 3 hours.
91% of subjects had septic shock; the remaining subjects had other forms of distributive shock such as neurogenic shock. At the time of study drug administration, 97% of subjects were receiving norepinephrine, 67% vasopressin, 15% phenylephrine, 13% epinephrine, and 2% dopamine. 83% of subjects had received two or more vasopressors and 47% three or more vasopressors prior to study drug administration. 61% of subjects were male, 80% were White, 10% were Black, and 10% were other races. The median age of subjects was 64 years (range: 22-89 years). Patients requiring high doses of steroids, patients with a history of asthma or bronchospasm, and patients with Raynaud's syndrome were not included.
The primary endpoint was achieved by 70% of patients randomized to GIAPREZA compared to 23% of placebo subjects; p < 0.0001 (a treatment effect of 47%). Figure 1 shows the results in all patients and in selected subgroups.
Figure 1: ATHOS-3: Primary Endpoint – Overall Result and Results in Selected Subgroups
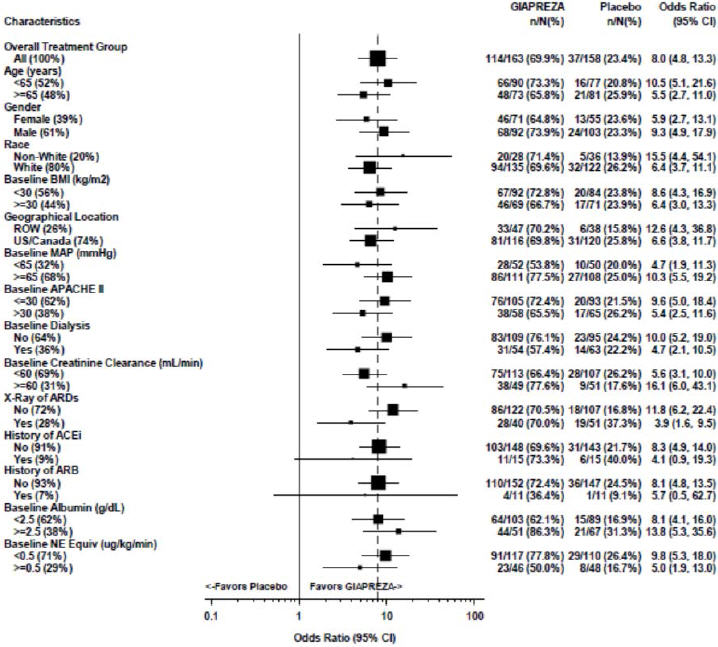
NE Equiv = norepinephrine equivalent dose: the sum of all vasopressors doses with each vasopressor dose converted to the clinically equivalent norepinephrine dose
Note: The figure above presents effects in various subgroups, all of which are baseline characteristics. The 95% confidence limits that are shown do not take into account the number of comparisons made, and may not reflect the effect of a particular factor after adjustment for all other factors. Apparent homogeneity or heterogeneity among groups should not be over-interpreted.
In the GIAPREZA-treated group, the median time to reach the target MAP endpoint was 5 minutes. The effect on MAP was sustained for at least the first three hours of treatment. The median dose of GIAPREZA was 10 ng/kg/min at 30 minutes. Of the 114 responders at Hour 3, only 2 (1.8%) received more than 80 ng/kg/min.
Patients were not necessarily on maximum doses of other vasopressors at the time of randomization. The effect of GIAPREZA when added to maximum doses of other vasopressors is unknown.
Mortality through Day 28 was 46% on GIAPREZA and 54% on placebo (hazard ratio 0.78; 95% confidence interval 0.57 – 1.07).
-
16. HOW SUPPLIED/STORAGE AND HANDLING
16.1. How Supplied
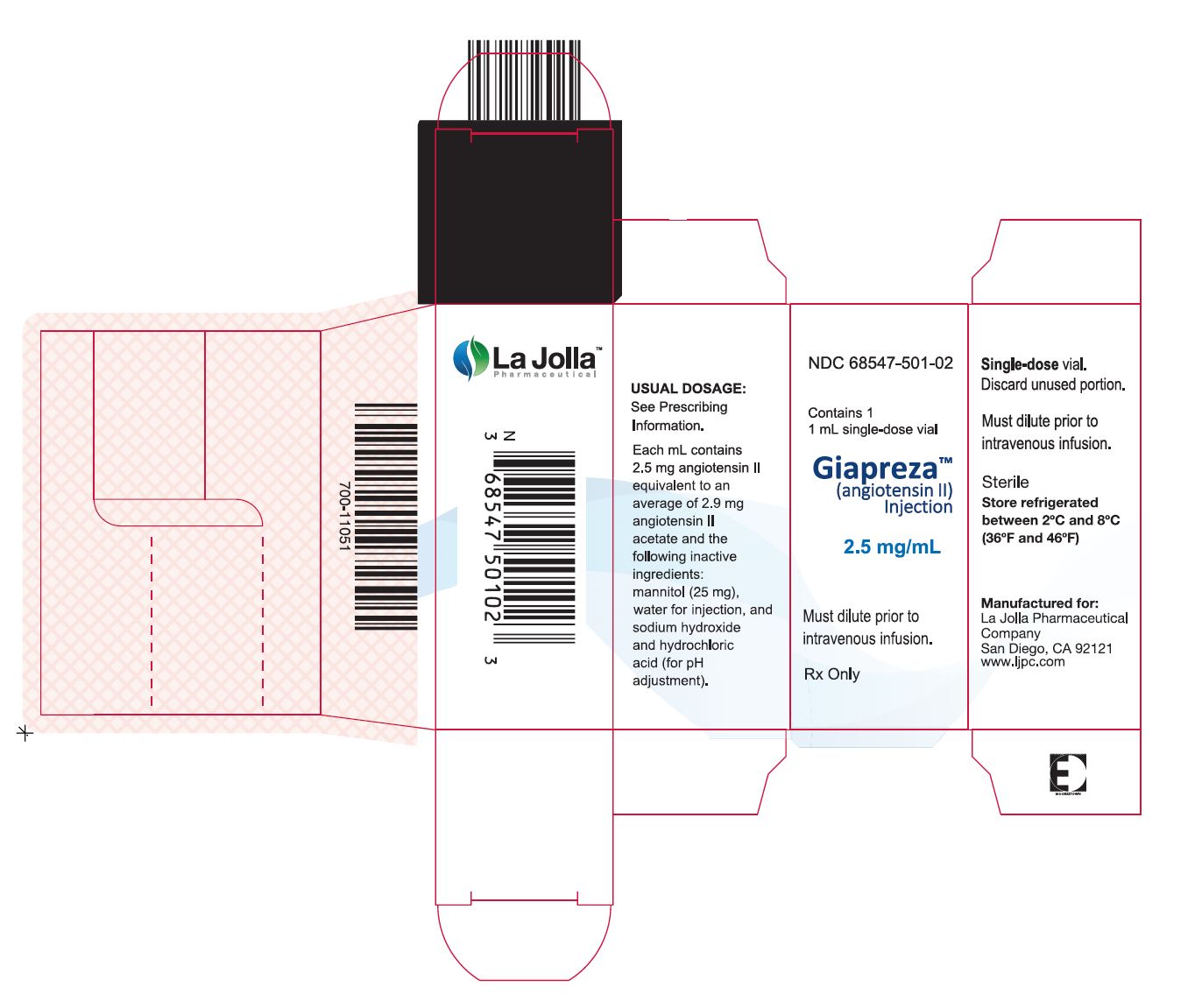
GIAPREZA (angiotensin II) Injection is a clear, aqueous solution for administration by intravenous infusion supplied as a single dose vial in two strengths:
- 2.5 mg/mL vial: NDC: 68547-501-02: A carton of one 1 mL single dose vial containing 2.5 mg angiotensin II (as a sterile liquid).
- 5 mg/2 mL vial: NDC: 68547-002-05: A carton of one 2 mL single dose vial containing 5 mg (2.5 mg/mL) angiotensin II (as a sterile liquid).
- SPL UNCLASSIFIED SECTION
- Package Label – 1 mL Single-Dose Vial Label
- Package Label – 1 mL Single Vial Carton Label
-
INGREDIENTS AND APPEARANCE
GIAPREZA
angiotensin ii injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68547-501 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANGIOTENSIN II (UNII: M089EFU921) (ANGIOTENSIN II - UNII:M089EFU921) ANGIOTENSIN II 2.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) 25 mg in 1 mL WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68547-501-02 1 in 1 CARTON 02/05/2018 1 1 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA209360 02/05/2018 Labeler - La Jolla Pharmaceutical Company (613541192) Establishment Name Address ID/FEI Business Operations Cangene Biopharma, LLC dba Emergent BioSolutions 050783398 manufacture(68547-501) , analysis(68547-501)
Trademark Results [Giapreza]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 GIAPREZA 87720479 5687263 Live/Registered |
La Jolla Pharmaceutical Company 2017-12-14 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.