DELFLEX- dextrose monohydrate, sodium chloride, sodium lactate, calcium chloride, magnesium chloride solution
DELFLEX by
Drug Labeling and Warnings
DELFLEX by is a Prescription medication manufactured, distributed, or labeled by Fresenius Medical Care North America. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
Description
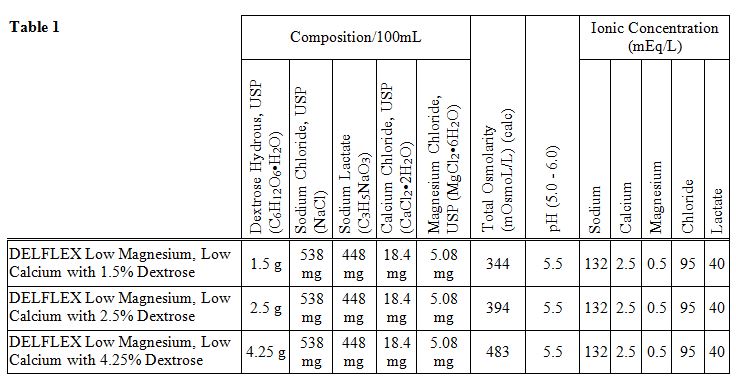
The DELFLEX ® peritoneal dialysis solutions (low magnesium/low calcium) are sterile, non-pyrogenic formulations of dextrose and electrolytes in water for injection, USP, for use in peritoneal dialysis. These solutions do not contain antimicrobial agents or additional buffers. The staysafe ® Exchange Set utilizes an easy to use dial designed to eliminate the use of clamps and to prevent touch contamination of internal connection components. Composition, calculated osmolarity, pH and ionic concentrations are shown in Table 1.
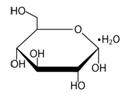
Dextrose, USP, is chemically designated D-glucose monohydrate (C 6H 12O 6H 2O) a hexose sugar freely soluble in water. The structural formula is shown here:
Calcium chloride, USP, is chemically designated calcium chloride dihydrate (CaCl 22H 2O) white fragments or granules freely soluble in water.
Magnesium chloride, USP, is chemically designated magnesium chloride hexahydrate (MgCl 26H 2O) colorless flakes or crystals very soluble in water.
Sodium lactate solution, USP, is chemically designated (CH 3CH(OH)COONa), a 60% aqueous solution miscible in water.
Sodium chloride, USP, is chemically designated (NaCl), a white, crystalline compound freely soluble in water.
Water for injection, USP, is chemically designated (H 2O).
Hydrochloric Acid or Sodium Hydroxide may be added for pH adjustment. pH is 5.5 ± 0.5.
Exposure to temperatures above 25°C(77°F) during transport and storage will lead to minor losses in moisture content. Higher temperatures lead to greater losses. It is unlikely that these minor losses will lead to clinically significant changes within the expiration period. Since the flexible inner bag is compounded from a specific polyvinyl chloride, water may permeate from the inner bag into the outerwrap in quantities insufficient to affect the solution significantly. Solutions in contact with the plastic inner bag can cause certain chemical components of the bag to leach out in very small amounts; however, the safety of the plastic formulation is supported by biological tests for plastic containers.
Table 1. Composition, Calculated Osmolarity, pH, and Ionic Concentration
-
Clinical Pharmacology
Peritoneal dialysis is the process of filtering excess water and toxins from the bloodstream through a semi-permeable membrane. This process does not cure the disease, but prevents progression of symptoms. Dialysis for chronic kidney failure is essential to maintain life, unless the patient receives a kidney transplant. A peritoneal dialysis procedure utilizes the peritoneum (lining of the abdomen) as the semi-permeable membrane. The procedure is conducted by instilling peritoneal dialysis solution through a catheter in the abdomen into the peritoneal cavity. Since the peritoneum is heavily supplied with blood vessels, the contact of the solution with the peritoneum causes excess water and toxins in the bloodstream to be drawn across the membrane into the solution. This osmosis and diffusion occurs between the plasma of the patient and the peritoneal dialysis solution. After a period of time called "dwell time," the solution is then drained from the patient.
This solution does not contain potassium. In situations in which there is a normal serum potassium level or hypokalemia, the addition of potassium chloride (up to a concentration of 4 mEq/L) may be indicated to prevent severe hypokalemia. Addition of potassium chloride should be made after careful evaluation of serum and total body potassium and only under the direction of a physician.
Clinical studies have demonstrated that the use of low magnesium solutions resulted in significant increases in serum CO 2 and decreases in serum magnesium levels. The decrease in magnesium levels did not cause clinically significant hypomagnesemia.
- Indications and Usage
- Contraindications
-
Warnings
Not for Intravenous Injection.
Use Aseptic Technique.
After removing the outerwrap, check for minute leaks by squeezing the solution bag firmly. If leaks are found, discard the solution because the sterility may be impaired. (A small amount of moisture may be present inside the outerwrap, which is normal condensation from the sterilization process).
Peritoneal dialysis should be done with great care, in patients with a number of conditions, including disruption of the peritoneal membrane or diaphragm by surgery or trauma, extensive adhesions, bowel distention, undiagnosed abdominal disease, abdominal wall infection, hernias or burns, fecal fistula or colostomy, tense ascites, obesity, large polycystic kidneys, recent aortic graft replacement, lactic acidosis and severe pulmonary disease. When assessing peritoneal dialysis as the mode of therapy in such extreme situations, the benefits to the patient must be weighed against the possible complications.
Solutions containing lactate ion should be used with great care in patients with metabolic or respiratory alkalosis. Lactate should be administered with great care in those conditions in which there is an increased level or an impaired utilization of this ion, such as severe hepatic insufficiency.
An accurate fluid balance record must be kept and the weight of the patient carefully monitored to avoid over or under hydration with severe consequences, including congestive heart failure, volume depletion and shock.
Excessive use of DELFLEX ® peritoneal dialysis solution with 4.25% dextrose during a peritoneal dialysis treatment can result in significant removal of water from the patient.
Stable patients undergoing maintenance peritoneal dialysis should have routine periodic evaluation of electrolyte blood chemistries and hematologic factors, as well as other indicators that determine the patient's ongoing status.
Serum calcium levels in patients using low calcium concentrations should be monitored and if found to be low, the peritoneal solution in use should be altered to one with a higher calcium concentration.
-
Precautions
General
Chronic patients that have been stabilized on peritoneal dialysis therapy should have routine evaluation of electrolyte blood chemistries and hematologic factors measured in order to determine the patient's ongoing condition.
DELFLEX ® peritoneal dialysis solutions do not include potassium. Potassium chloride should only be added under the direction of a physician after careful evaluation of both serum and total body potassium.
Significant loss of protein, amino acids and water soluble vitamins may occur during peritoneal dialysis. Replacement therapy should be provided as necessary.
Information for Patients
Aseptic technique must be used throughout the procedure and at its termination in order to reduce the possibility of infection.
The outerwrap should remain intact until time of use.
Administer only if the solution is clear, all seals are intact, and there is no evidence of leaking.
Do not heat in a microwave oven. Microwave ovens heat unevenly and can leave hot spots, which can burn the peritoneum.
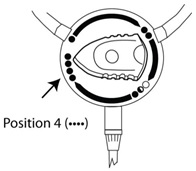
Disconnect from disk only when knob is in position 4 () to ensure patient connector is sealed.
Care should be taken to ensure that there is not any leakage around the catheter, since if not controlled, the leakage can create edema from subcutaneous infiltration of the dialysis solution. The leakage will also create an inaccurate fluid balance measurement. If any leakage is identified do not proceed with infusion and notify your physician.
Laboratory Tests
Serum electrolytes, magnesium, bicarbonate levels and fluid balance should be periodically monitored.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long term animal studies with DELFLEX ® peritoneal dialysis solutions have not been performed to evaluate the carcinogenic potential, mutagenic potential or effect on fertility.
Pregnancy: Teratology Effects
Pregnancy Category C. Animal reproduction studies have not been conducted with DELFLEX ® peritoneal dialysis solutions. It is also not known whether DELFLEX ® peritoneal dialysis solutions can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. DELFLEX ® peritoneal dialysis solutions should be given to a pregnant woman only if clearly needed.
Nursing Mothers
Caution should be exercised when DELFLEX ® peritoneal dialysis solutions are administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
-
Adverse Reactions
Adverse reactions occurring with administration of peritoneal dialysis include mechanical and solution related problems as well as the results of contamination of equipment or improper technique in catheter placement. Abdominal pain, bleeding, peritonitis, subcutaneous infection around a peritoneal catheter, catheter blockage, difficulty in fluid removal, and ileus are among the complications of the procedure. Solution related adverse reactions may include peritonitis, catheter site infection, electrolyte and fluid imbalances, hypovolemia, hypervolemia, hypertension, hypotension, disequilibrium syndrome and muscle cramping.
If an adverse reaction does occur, institute appropriate therapeutic procedures according to the patient's needs and conditions, and save the remainder of the fluid in the bag for evaluation if deemed necessary.
-
Dosage And Administration
DELFLEX ® peritoneal dialysis solutions are provided for intraperitoneal administration only. The mode of therapy, frequency of treatment, formulation, exchange volume, duration of dwell, and length of dialysis should be selected by the physician responsible for the treatment of the individual patient.
To avoid the risk of severe dehydration or hypovolemia and to minimize the loss of protein, it is advisable to select the peritoneal dialysis solution with lowest level of osmolarity consistent with the fluid removal requirements for that exchange.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
Additives may be incompatible. Please refer to manufacturer’s product insert. Do not store solutions containing additives.
For administration see Directions for Use section.
-
How Supplied
DELFLEX ® peritoneal dialysis solutions are delivered in single-dose flexible bags. All DELFLEX ® peritoneal dialysis solutions have overfills declared on the bag label. The flexible bag has the capacity for drainage in excess of their stated fill volume for ultrafiltration from the patient.
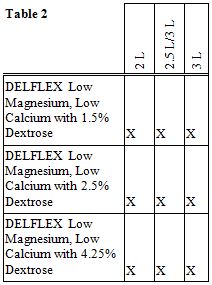
DELFLEX ® peritoneal dialysis solutions with an attached staysafe ® Exchange Set are available in the sizes and formulations shown in Table 2.
Storage Conditions
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (between 59°F and 86°F). See USP Controlled Room Temperature. Brief exposure to temperatures up to 40°C (104°F) may be tolerated provided the mean kinetic temperature does not exceed 25°C (77°F); however, such exposure should be minimized.
Keep DELFLEX ® and all medicines out of the reach of children.
Not for Intravenous Injection. Do not microwave.
Warm solution as directed by your health care provider.Directions for Use (Aseptic technique is required)
Get Ready
- Clean work surface.
- Gather supplies:
- DELFLEX ® Peritoneal Dialysis bag with attached staysafe ® Exchange Set.
- Povidone iodine prefilled staysafe ® cap, a stand alone item provided separately.
- staysafe ® Organizer, a stand alone item provided separately (Optional; Fresenius Medical Care North America (FMCNA) recommends its use).
- Prescribed medication(s), if ordered by your healthcare provider.
- Mask.
- Put on mask. Wash your hands.
- Ensure that the Extension Set coming from your catheter is clamped.
- Tear the outerwrap from the slit edge down the length of the inner bags to open. Wipe away any moisture from the solution bag. Some opacity may be observed in the plastic of the bag and/or tubing and is due to moisture absorption during the sterilization process. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually.
Inspect DELFLEX ® Solution Bag: - Place the DELFLEX ® solution set on the work surface. Separate the fill and drain bag.
- Visually inspect the solution to ensure that it is clear and free of particulate matter prior to administration. Color may vary from clear to slightly yellow but does not affect efficacy and may be used.
- Check the expiration date. Check for correct dextrose concentration.
- Firmly squeeze the Solution Bag to check for leaks.
Do not use DELFLEX ® solution if:- Leaks are found
- The solution bag is damaged
- Solution is cloudy or discolored
Note: Retain DELFLEX ® peritoneal dialysis bag sample for manufacturer evaluation and notify your healthcare provider if any of the above defects are found.
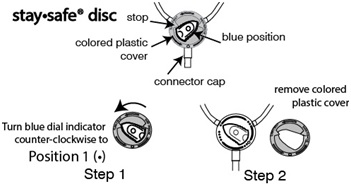
- Turn the blue position indicator on the staysafe ® disc counter-clockwise until it fits into the cut-out portion of the colored plastic cover on the disc. See Figure A, Step 1. Remove the colored plastic cover while the indicator is in this position (Position 1: ). See Figure A, Step 2. Once the cover is removed, do not turn counter-clockwise. (This step is done in preparation to allow the fluid in your peritoneal cavity to drain later on in this procedure).
Figure A
Administer DELFLEX ® Peritoneal Dialysis Solution
- If you will be adding medication(s):
- Clean the medication port as instructed by your healthcare provider.
- Add the medicine(s).
- Turn the bag upside down several times to mix the medicine(s).
- Hang the solution bag from the I.V. pole. Place the drain bag at floor level.
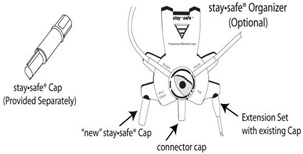
- Break the frangible in the solution bag outlet port. (If using the Organizer, place the staysafe
® disc in the Organizer as illustrated in Figure B)
Figure B
- Remove the new staysafe ® Cap from its package. (The new staysafe ® Cap is the stand alone item provided to the patient separately). (If using the Organizer, place the new staysafe ® Cap in the left notch of the Organizer. Place the existing cap of staysafe ® Extension Set, connected to the patient’s catheter, in the other notch of the Organizer). See Figure B.
- Aseptically remove the connector cap from the staysafe ® disc and throw the cap away. Remove the existing cap from the Extension Set connected to the patient’s catheter by twisting the connection counter-clockwise. (If using the Organizer, leave the capped end of the Extension Set in the Organizer and twist the Extension Set connector counter-clockwise to remove the set from its cap.)
- Aseptically connect the Extension Set to the connector on the staysafe ® disc. Twist clockwise to secure the connection.
- Remove your mask. Do not open the system during exchange.
- Open the Extension Set clamp to start drain.
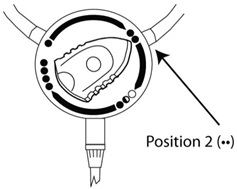
- When patient drain is complete, turn the staysafe
® disc position indicator to Position 2 (). See
Figure C. This will start flush from the solution bag to the drain bag.
Figure C
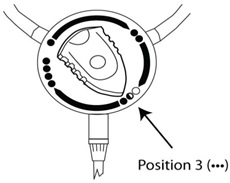
- After approximately 5 seconds turn the staysafe
® disc position indicator to Position 3 (). See
Figure D. This will start the patient fill.
Figure D
- When fill is complete, turn the staysafe
® disc position indicator to Position 4 (). See
Figure E. This will insert the closure pin of the disc into the Extension Set connector and seal the system.
Figure E
- Close the clamp on the Extension Set. Remove the white protective cover from the new staysafe ® Cap. Save for later use.
- Remove the Extension Set from the staysafe ® disc and attach the new staysafe ® Cap. Twist clockwise to secure the connection.
- Seal the disc by attaching the white protective cover from the new staysafe ® Cap to the disc connector. Twist clockwise to secure the connection and prevent leakage from the used system.
- Look at the drained fluid for cloudiness. Measure the amount of fluid drained. Throw away the fluid and used set as instructed by your healthcare provider. In case of cloudiness, save the fluid and the used set and immediately contact your healthcare provider.
Fresenius Medical Care North America
920 Winter Street
Waltham, MA 024511-800-323-5188
Fresenius Medical Care, triangle logo, staysafe, Delflex, Safe-Lock are trademarks of Fresenius Medical Care Holding, Inc. or its affiliated companies.
89-908-85 REV 02/15
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DELFLEX
dextrose monohydrate, sodium chloride, sodium lactate, calcium chloride, magnesium chloride solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 49230-206 Route of Administration INTRAPERITONEAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 1.5 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 538 mg in 100 mL SODIUM LACTATE (UNII: TU7HW0W0QT) (SODIUM CATION - UNII:LYR4M0NH37, LACTIC ACID - UNII:33X04XA5AT) SODIUM LACTATE 448 mg in 100 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) (CALCIUM CATION - UNII:2M83C4R6ZB, CHLORIDE ION - UNII:Q32ZN48698) CALCIUM CHLORIDE 18.4 mg in 100 mL MAGNESIUM CHLORIDE (UNII: 02F3473H9O) (MAGNESIUM CATION - UNII:T6V3LHY838, CHLORIDE ION - UNII:Q32ZN48698) MAGNESIUM CHLORIDE 5.08 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49230-206-92 5 in 1 CARTON 08/19/1992 1 2000 mL in 1 BAG; Type 0: Not a Combination Product 2 NDC: 49230-206-94 5 in 1 CARTON 08/19/1992 2 2500 mL in 1 BAG; Type 0: Not a Combination Product 3 NDC: 49230-206-95 4 in 1 CARTON 08/19/1992 3 3000 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020171 08/19/1992 DELFLEX
dextrose monohydrate, sodium chloride, sodium lactate, calcium chloride, magnesium chloride solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 49230-209 Route of Administration INTRAPERITONEAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 2.5 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 538 mg in 100 mL SODIUM LACTATE (UNII: TU7HW0W0QT) (SODIUM CATION - UNII:LYR4M0NH37, LACTIC ACID - UNII:33X04XA5AT) SODIUM LACTATE 448 mg in 100 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) (CALCIUM CATION - UNII:2M83C4R6ZB, CHLORIDE ION - UNII:Q32ZN48698) CALCIUM CHLORIDE 18.4 mg in 100 mL MAGNESIUM CHLORIDE (UNII: 02F3473H9O) (MAGNESIUM CATION - UNII:T6V3LHY838, CHLORIDE ION - UNII:Q32ZN48698) MAGNESIUM CHLORIDE 5.08 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49230-209-92 5 in 1 CARTON 08/19/1992 1 2000 mL in 1 BAG; Type 0: Not a Combination Product 2 NDC: 49230-209-94 5 in 1 CARTON 08/19/1992 2 2500 mL in 1 BAG; Type 0: Not a Combination Product 3 NDC: 49230-209-95 4 in 1 CARTON 08/19/1992 3 3000 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020171 08/19/1992 DELFLEX
dextrose monohydrate, sodium chloride, sodium lactate, calcium chloride, magnesium chloride solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 49230-212 Route of Administration INTRAPERITONEAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 4.25 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 538 mg in 100 mL SODIUM LACTATE (UNII: TU7HW0W0QT) (SODIUM CATION - UNII:LYR4M0NH37, LACTIC ACID - UNII:33X04XA5AT) SODIUM LACTATE 448 mg in 100 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) (CALCIUM CATION - UNII:2M83C4R6ZB, CHLORIDE ION - UNII:Q32ZN48698) CALCIUM CHLORIDE 18.4 mg in 100 mL MAGNESIUM CHLORIDE (UNII: 02F3473H9O) (MAGNESIUM CATION - UNII:T6V3LHY838, CHLORIDE ION - UNII:Q32ZN48698) MAGNESIUM CHLORIDE 5.08 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49230-212-92 5 in 1 CARTON 08/19/1992 1 2000 mL in 1 BAG; Type 0: Not a Combination Product 2 NDC: 49230-212-94 5 in 1 CARTON 08/19/1992 2 2500 mL in 1 BAG; Type 0: Not a Combination Product 3 NDC: 49230-212-95 4 in 1 CARTON 08/19/1992 3 3000 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020171 08/19/1992 Labeler - Fresenius Medical Care North America (958291411)
Trademark Results [DELFLEX]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 DELFLEX 78560327 3054723 Live/Registered |
Fresenius Medical Care Holdings, Inc. 2005-02-03 |
 DELFLEX 74094930 1687522 Dead/Cancelled |
Delmed Inc. 1990-09-06 |
 DELFLEX 72352463 0922661 Dead/Expired |
DELTA STRAPPING INDUSTRIES, INC. 1970-02-26 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.