RELIBIOTIC- lactobacillus acidophilus and bifidobacterium animalis lactis capsule
ReliBiotic by
Drug Labeling and Warnings
ReliBiotic by is a Other medication manufactured, distributed, or labeled by Redmont Pharmaceuticals, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- STATEMENT OF IDENTITY
- DESCRIPTION
- STATEMENT OF IDENTITY
-
INDICATIONS
ReliBiotic is an orally administered prescription probiotic formulation for the clinical dietary management of suboptimal nutritional status in patients where advanced supplementation is required and nutritional supplementation in physiologically stressful conditions for maintenance of good health is needed. ReliBiotic should be administered under the supervision of a licensed medical practitioner.
- CONTRAINDICATIONS
- WARNING
- ADVERSE REACTIONS
- DOSAGE AND ADMINISTRATION
-
HOW SUPPLIED
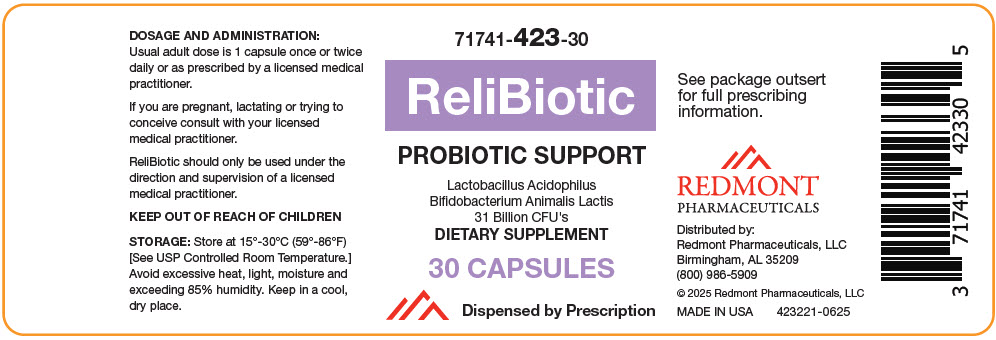
Bottle of 30 capsules (71741-423-301). Capsule is off-white, oblong.
- 1 Redmont Pharmaceuticals does not represent this product code to be National Drug Code (NDC). Product codes are formatted according to standard industry practice, to meet the formatting requirement by pedigree reporting and supply-chain control including pharmacies.
- STORAGE AND HANDLING
- HEALTH CLAIM
- SAFE HANDLING WARNING
- HEALTH CLAIM
- PRINCIPAL DISPLAY PANEL - 30 Capsule Bottle Label
-
INGREDIENTS AND APPEARANCE
RELIBIOTIC
lactobacillus acidophilus and bifidobacterium animalis lactis capsuleProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:71741-423 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Lactobacillus Acidophilus (UNII: 1PRR1V42V5) (Lactobacillus Acidophilus - UNII:1PRR1V42V5) Lactobacillus Acidophilus 16000000000 [CFU] Bifidobacterium Animalis Lactis (UNII: 5307V7XW8I) (Bifidobacterium Animalis Lactis - UNII:5307V7XW8I) Bifidobacterium Animalis Lactis 15000000000 [CFU] Inactive Ingredients Ingredient Name Strength HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) Silicon Dioxide (UNII: ETJ7Z6XBU4) MAGNESIUM PALMITOSTEARATE (UNII: R4OXA9G5BV) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:71741-423-30 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT 05/20/2025 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color scoring 1 shape size (solid drugs) 23 mm Labeler - Redmont Pharmaceuticals, LLC (080843607)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.