Aspirin 81 mg by AACE Pharmaceuticals, Inc.
Aspirin 81 mg by
Drug Labeling and Warnings
Aspirin 81 mg by is a Otc medication manufactured, distributed, or labeled by AACE Pharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ASPIRIN 81 MG- aspirin enteric coated tablets 81 mg tablet, delayed release
AACE Pharmaceuticals, Inc.
----------
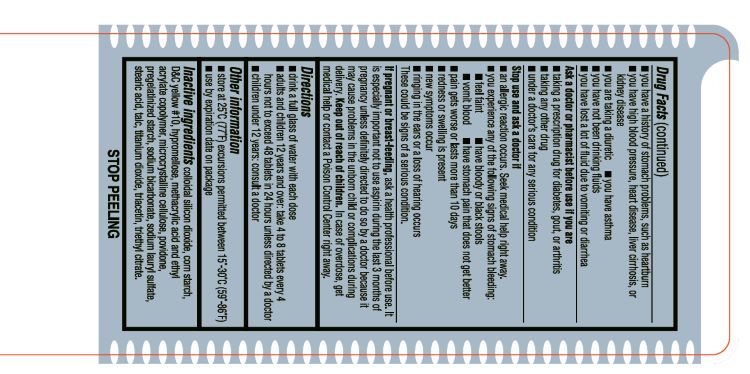
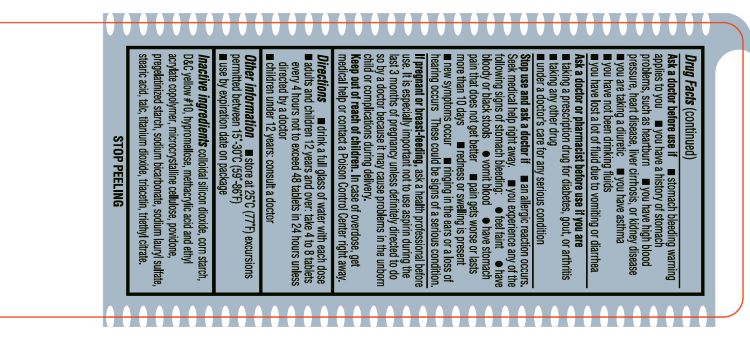
Aspirin Drug Facts
Do not use: if you are allergic to aspirin or any other pain reliver/fever reducer
 NDC: 71406-128-12 AACE Pharmaceuticals - Adult Low Strength - Pain Reliever - Aspirin USP 81 mg (NSAID) Enteric Coated - 120 tablets - Compare to Active Ingredient in Aspirin Regimen BAYER®
NDC: 71406-128-12 AACE Pharmaceuticals - Adult Low Strength - Pain Reliever - Aspirin USP 81 mg (NSAID) Enteric Coated - 120 tablets - Compare to Active Ingredient in Aspirin Regimen BAYER®

NDC: 71406-128-10 AACE Pharmaceuticals - Adult Low Strength - Pain Reliever - Aspirin USP 81 mg (NSAID) Enteric Coated - 1000 tablets - Compare to Active Ingredient in Aspirin Regimen BAYER®

| ASPIRIN 81 MG
aspirin enteric coated tablets 81 mg tablet, delayed release |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - AACE Pharmaceuticals, Inc. (080630748) |
Revised: 5/2025
Document Id: 351de7c2-98c2-7c88-e063-6294a90ad811
Set id: c3635e98-93cf-0e1e-e053-2a95a90ab7b9
Version: 8
Effective Time: 20250514