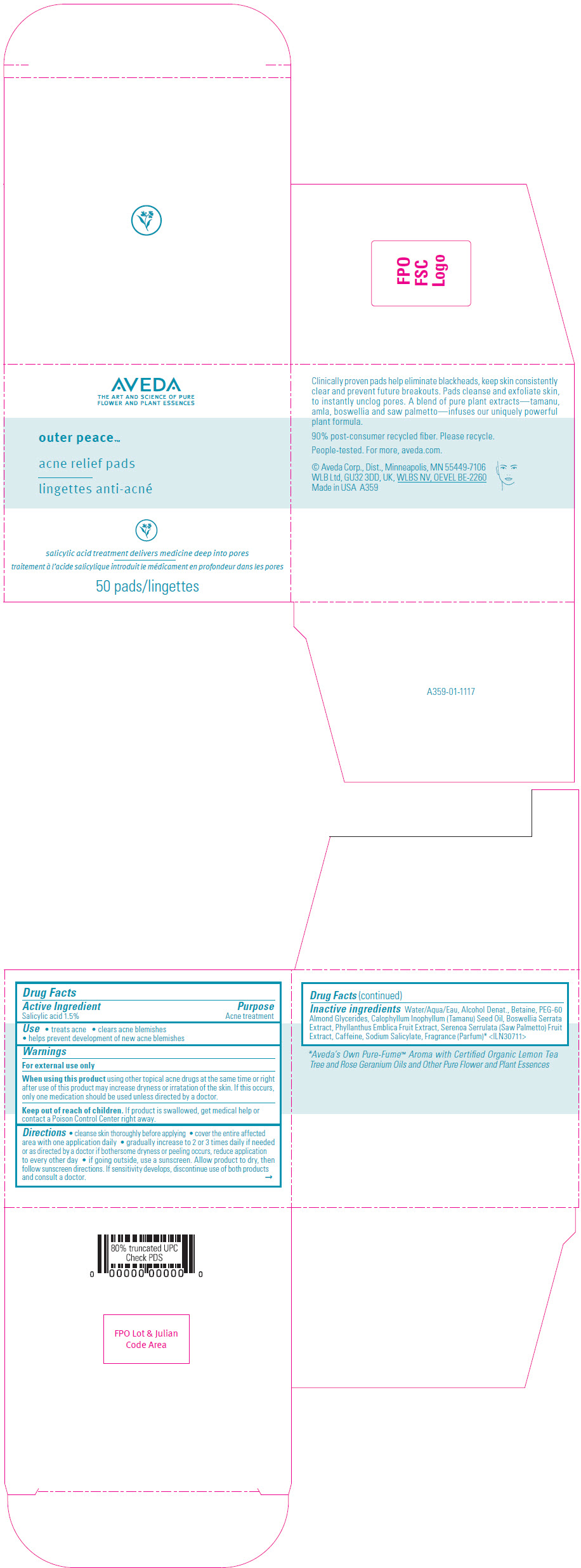
AVEDA OUTER PEACE ACNE RELIEF PADS- salicylic acid solution
AVEDA OUTER PEACE ACNE RELIEF PADS by
Drug Labeling and Warnings
AVEDA OUTER PEACE ACNE RELIEF PADS by is a Otc medication manufactured, distributed, or labeled by Aveda Corporation, Estee Lauder Companies Inc., Port Jervis Laboratories, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Use
- Warnings
-
Directions
- cleanse skin thoroughly before applying
- cover the entire affected area with one application daily
- gradually increase to 2 or 3 times daily if needed or as directed by a doctor if bothersome dryness or peeling occurs, reduce application to every other day
- if going outside, use a sunscreen. Allow product to dry, then follow sunscreen directions. If sensitivity develops, discontinue use of both products and consult a doctor.
-
Inactive ingredients
Water/Aqua/Eau, Alcohol Denat., Betaine, PEG-60 Almond Glycerides, Calophyllum Inophyllum (Tamanu) Seed Oil, Boswellia Serrata Extract, Phyllanthus Emblica Fruit Extract, Serenoa Serrulata (Saw Palmetto) Fruit Extract, Caffeine, Sodium Salicylate, Fragrance (Parfum) 1 <ILN30711>
- 1 Aveda's Own Pure-Fume™ Aroma with Certified Organic Lemon Tea Tree and Rose Geranium Oils and Other Pure Flower and Plant Essences
- PRINCIPAL DISPLAY PANEL - 50 Pad Jar Carton
-
INGREDIENTS AND APPEARANCE
AVEDA OUTER PEACE ACNE RELIEF PADS
salicylic acid solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 57677-064 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 15 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) BETAINE (UNII: 3SCV180C9W) PEG-60 ALMOND GLYCERIDES (UNII: 4Y0E651N0F) TAMANU OIL (UNII: JT3LVK84A1) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) PHYLLANTHUS EMBLICA FRUIT (UNII: YLX4CW2576) SAW PALMETTO (UNII: J7WWH9M8QS) CAFFEINE (UNII: 3G6A5W338E) SODIUM SALICYLATE (UNII: WIQ1H85SYP) FRAGRANCE CLEAN ORC0600327 (UNII: 329LCV5BTF) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 57677-064-01 1 in 1 CARTON 05/17/2021 1 50 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 05/17/2021 Labeler - Aveda Corporation (071352058) Registrant - Estee Lauder Companies Inc. (790802086) Establishment Name Address ID/FEI Business Operations Port Jervis Laboratories, Inc 001535103 manufacture(57677-064)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.