COTELLIC- cobimetinib tablet, film coated
Cotellic by
Drug Labeling and Warnings
Cotellic by is a Prescription medication manufactured, distributed, or labeled by Genentech, Inc., F. Hoffmann-La Roche AG. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use COTELLIC safely and effectively. See full prescribing information for COTELLIC.
COTELLIC® (cobimetinib) tablets, for oral use
Initial U.S. Approval: 2015INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Tablets: 20 mg (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- New primary malignancies, cutaneous and non-cutaneous: Monitor patients for new malignancies prior to initiation of therapy, while on therapy, and for up to 6 months following the last dose of COTELLIC. (5.1)
- Hemorrhage: Major hemorrhagic events can occur with COTELLIC. Monitor for signs and symptoms of bleeding. (5.2, 2.3)
- Cardiomyopathy: The risk of cardiomyopathy is increased in patients receiving COTELLIC with vemurafenib compared with vemurafenib as a single agent. The safety of COTELLIC has not been established in patients with decreased left ventricular ejection fraction (LVEF). Evaluate LVEF before treatment, after one month of treatment, then every 3 months thereafter during treatment with COTELLIC. (5.3, 2.3)
- Severe Dermatologic Reactions: Monitor for severe skin rashes. Interrupt, reduce, or discontinue COTELLIC. (5.4, 2.3)
- Serous Retinopathy and Retinal Vein Occlusion: Perform an ophthalmological evaluation at regular intervals and for any visual disturbances. Permanently discontinue COTELLIC for retinal vein occlusion (RVO). (5.5, 2.3)
- Hepatotoxicity: Monitor liver laboratory tests during treatment and as clinically indicated. (5.6, 2.3)
- Rhabdomyolysis: Monitor creatine phosphokinase periodically and as clinically indicated for signs and symptoms of rhabdomyolysis. (5.7, 2.3)
- Severe Photosensitivity: Advise patients to avoid sun exposure. (5.8, 2.3)
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and to use effective contraception. (5.9, 8.1, 8.3)
ADVERSE REACTIONS
Most common adverse reactions for COTELLIC (≥20%) are diarrhea, photosensitivity reaction, nausea, pyrexia, and vomiting. The most common (≥5%) Grade 3-4 laboratory abnormalities are increased GGT, increased CPK, hypophosphatemia, increased ALT, lymphopenia, increased AST, increased alkaline phosphatase, hyponatremia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Genentech at 1-888-835-2555 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 1/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Patient Selection
2.2 Recommended Dose
2.3 Dose Modifications
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 New Primary Malignancies
5.2 Hemorrhage
5.3 Cardiomyopathy
5.4 Severe Dermatologic Reactions
5.5 Serous Retinopathy and Retinal Vein Occlusion
5.6 Hepatotoxicity
5.7 Rhabdomyolysis
5.8 Severe Photosensitivity
5.9 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Effect of Strong or Moderate CYP3A Inhibitors on Cobimetinib
7.2 Effect of Strong or Moderate CYP3A Inducers on Cobimetinib
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Patient Selection
Confirm the presence of BRAF V600E or V600K mutation in tumor specimens prior to initiation of treatment with COTELLIC with vemurafenib. Information on FDA-approved tests for the detection of BRAF V600 mutations in melanoma is available at: http://www.fda.gov/CompanionDiagnostics.
2.2 Recommended Dose
The recommended dosage regimen of COTELLIC is 60 mg (three 20 mg tablets) orally taken once daily for the first 21 days of each 28-day cycle until disease progression or unacceptable toxicity [see Clinical Studies (14)].
Take COTELLIC with or without food [see Clinical Pharmacology (12.3)].
If a dose of COTELLIC is missed or if vomiting occurs when the dose is taken, resume dosing with the next scheduled dose.
2.3 Dose Modifications
Concurrent CYP3A Inhibitors
Do not take strong or moderate CYP3A inhibitors while taking COTELLIC.
If concurrent short term (14 days or less) use of moderate CYP3A inhibitors is unavoidable for patients who are taking COTELLIC 60 mg, reduce COTELLIC dose to 20 mg. After discontinuation of a moderate CYP3A inhibitor, resume previous dose of COTELLIC 60 mg [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
Use an alternative to a strong or moderate CYP3A inhibitor in patients who are taking a reduced dose of COTELLIC (40 or 20 mg daily) [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
Adverse Reactions
Review the Full Prescribing Information for vemurafenib for recommended dose modifications.
Table 1. Recommended Dose Reductions for COTELLIC First Dose Reduction 40 mg orally once daily Second Dose Reduction 20 mg orally once daily Subsequent Modification Permanently discontinue COTELLIC if unable to tolerate 20 mg orally once daily Table 2. Recommended Dose Modifications for COTELLIC for Adverse Reactions Severity of Adverse Reaction* Dose Modification for COTELLIC - * National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 (NCI CTCAE v4.0)
New Primary Malignancies (cutaneous and non-cutaneous) No dose modification is required. Hemorrhage Grade 3 Withhold COTELLIC for up to 4 weeks. - If improved to Grade 0 or 1, resume at the next lower dose level.
- If not improved within 4 weeks, permanently discontinue.
Grade 4 Permanently discontinue. Cardiomyopathy Asymptomatic, absolute decrease in LVEF from baseline of greater than 10% and less than institutional lower limit of normal (LLN) Withhold COTELLIC for 2 weeks; repeat LVEF.
Resume at next lower dose if all of the following are present- LVEF is at or above LLN and
- Absolute decrease from baseline LVEF is 10% or less.
Permanently discontinue if any of the following are present
- LVEF is less than LLN or
- Absolute decrease from baseline LVEF is more than 10%.
Symptomatic LVEF decrease from baseline Withhold COTELLIC for up to 4 weeks, repeat LVEF.
Resume at next lower dose if all of the following are present:- Symptoms resolve and
- LVEF is at or above LLN and
- Absolute decrease from baseline LVEF is 10% or less.
Permanently discontinue if any of the following are present
- Symptoms persist, or
- LVEF is less than LLN, or
- Absolute decrease from baseline LVEF is more than 10%.
Dermatologic Reactions Grade 2 (intolerable), Grade 3 or 4 Withhold or reduce dose. Serous Retinopathy or Retinal Vein Occlusion Serous retinopathy Withhold COTELLIC for up to 4 weeks. - If signs and symptoms improve, resume at the next lower dose level.
- If not improved or symptoms recur at the lower dose within 4 weeks, permanently discontinue.
Retinal vein occlusion Permanently discontinue COTELLIC. Liver Laboratory Abnormalities and Hepatotoxicity First occurrence Grade 4 Withhold COTELLIC for up to 4 weeks. - If improved to Grade 0 or 1, then resume at the next lower dose level.
- If not improved to Grade 0 or 1 within 4 weeks, permanently discontinue.
Recurrent Grade 4 Permanently discontinue COTELLIC. Rhabdomyolysis and Creatine Phosphokinase (CPK) elevations - Grade 4 CPK elevation
- Any CPK elevation and myalgia
Withhold COTELLIC for up to 4 weeks. - If improved to Grade 3 or lower, resume at the next lower dose level.
- If not improved within 4 weeks, permanently discontinue.
Photosensitivity Grade 2 (intolerable), Grade 3 or Grade 4 Withhold COTELLIC for up to 4 weeks. - If improved to Grade 0 or 1, resume at the next lower dose level.
- If not improved within 4 weeks, permanently discontinue.
Other - Grade 2 (intolerable) adverse reactions
- Any Grade 3 adverse reactions
Withhold COTELLIC for up to 4 weeks. - If improved to Grade 0 or 1, resume at the next lower dose level.
- If not improved within 4 weeks, permanently discontinue.
First occurrence of any Grade 4 adverse reaction - Withhold COTELLIC until adverse reaction improves to Grade 0 or 1. Then resume at the next lower dose level, OR
- Permanently discontinue.
Recurrent Grade 4 adverse reaction Permanently discontinue COTELLIC. - 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
Review the Full Prescribing Information for vemurafenib for information on the serious risks of vemurafenib.
5.1 New Primary Malignancies
New primary malignancies, cutaneous and non-cutaneous, can occur with COTELLIC.
Cutaneous Malignancies:
In Trial 1, the following cutaneous malignancies or premalignant conditions occurred in the COTELLIC with vemurafenib arm and the vemurafenib arm, respectively: cutaneous squamous cell carcinoma (cuSCC) or keratoacanthoma (KA) (6% and 20%), basal cell carcinoma (4.5% and 2.4%), and second primary melanoma (0.8% and 2.4%). Among patients receiving COTELLIC with vemurafenib, the median time to detection of first cuSCC/KA was 4 months (range: 2 to 11 months), and the median time to detection of basal cell carcinoma was 4 months (range: 27 days to 13 months). The time to onset in the two patients with second primary melanoma was 9 months and 12 months.
Perform dermatologic evaluations prior to initiation of therapy and every 2 months while on therapy. Manage suspicious skin lesions with excision and dermatopathologic evaluation. No dose modifications are recommended for COTELLIC [see Dosage and Administration (2.3)]. Conduct dermatologic monitoring for 6 months following discontinuation of COTELLIC when administered with vemurafenib.
Non-Cutaneous Malignancies:
Based on its mechanism of action, vemurafenib may promote growth and development of malignancies [refer to the Full Prescribing Information for vemurafenib]. In Trial 1, 0.8% of patients in the COTELLIC with vemurafenib arm and 1.2% of patients in the vemurafenib arm developed non-cutaneous malignancies.
Monitor patients receiving COTELLIC, when administered with vemurafenib, for signs or symptoms of non-cutaneous malignancies.
5.2 Hemorrhage
Hemorrhage, including major hemorrhages defined as symptomatic bleeding in a critical area or organ, can occur with COTELLIC.
In Trial 1, the incidence of Grade 3–4 hemorrhages was 1.2% in patients receiving COTELLIC with vemurafenib and 0.8% in patients receiving vemurafenib. Hemorrhage (all grades) was 13% in patients receiving COTELLIC with vemurafenib and 7% in patients receiving vemurafenib. Cerebral hemorrhage occurred in 0.8% of patients receiving COTELLIC with vemurafenib and in none of the patients receiving vemurafenib. Gastrointestinal tract hemorrhage (3.6% vs 1.2%), reproductive system hemorrhage (2.0% vs 0.4%), and hematuria (2.4% vs 0.8%) also occurred at a higher incidence in patients receiving COTELLIC with vemurafenib compared with patients receiving vemurafenib.
Withhold COTELLIC for Grade 3 hemorrhagic events. If improved to Grade 0 or 1 within 4 weeks, resume COTELLIC at a lower dose level. Discontinue COTELLIC for Grade 4 hemorrhagic events and any Grade 3 hemorrhagic events that do not improve [see Dosage and Administration (2.3)].
5.3 Cardiomyopathy
Cardiomyopathy, defined as symptomatic and asymptomatic decline in left ventricular ejection fraction (LVEF), can occur with COTELLIC. The safety of COTELLIC has not been established in patients with a baseline LVEF that is either below institutional lower limit of normal (LLN) or below 50%.
In Trial 1, patients were assessed for decreases in LVEF by echocardiograms or MUGA at baseline, Week 5, Week 17, Week 29, Week 43, and then every 4 to 6 months thereafter while receiving treatment. Grade 2 or 3 decrease in LVEF occurred in 26% of patients receiving COTELLIC with vemurafenib and 19% of patients receiving vemurafenib. The median time to first onset of LVEF decrease was 4 months (range 23 days to 13 months). Of the patients with decreased LVEF, 22% had dose interruption and/or reduction and 14% required permanent discontinuation. Decreased LVEF resolved to above the LLN or within 10% of baseline in 62% of patients receiving COTELLIC with a median time to resolution of 3 months (range: 4 days to 12 months).
Evaluate LVEF prior to initiation, 1 month after initiation, and every 3 months thereafter until discontinuation of COTELLIC. Manage events of left ventricular dysfunction through treatment interruption, reduction, or discontinuation [see Dosage and Administration (2.3)]. In patients restarting COTELLIC after a dose reduction or interruption, evaluate LVEF at approximately 2 weeks, 4 weeks, 10 weeks, and 16 weeks, and then as clinically indicated.
5.4 Severe Dermatologic Reactions
Severe rash and other skin reactions can occur with COTELLIC.
In Trial 1, Grade 3 to 4 rash, occurred in 16% of patients receiving COTELLIC with vemurafenib and in 17% of patients receiving vemurafenib, including Grade 4 rash in 1.6% of patients receiving COTELLIC with vemurafenib and 0.8% of the patients receiving vemurafenib. The incidence of rash resulting in hospitalization was 3.2% in patients receiving COTELLIC with vemurafenib and 2.0% in patients receiving vemurafenib. In patients receiving COTELLIC, the median time to onset of Grade 3 or 4 rash events was 11 days (range: 3 days to 2.8 months). Among patients with Grade 3 or 4 rash events, 95% experienced complete resolution with the median time to resolution of 21 days (range 4 days to 17 months).
Interrupt, reduce the dose, or discontinue COTELLIC [see Dosage and Administration (2.3)].
5.5 Serous Retinopathy and Retinal Vein Occlusion
Ocular toxicities can occur with COTELLIC, including serous retinopathy (fluid accumulation under layers of the retina).
In Trial 1, ophthalmologic examinations including retinal evaluation were performed pretreatment and at regular intervals during treatment. Symptomatic and asymptomatic serous retinopathy was identified in 26% of patients receiving COTELLIC with vemurafenib. The majority of these events were reported as chorioretinopathy (13%) or retinal detachment (12%). The time to first onset of serous retinopathy events ranged between 2 days to 9 months. The reported duration of serous retinopathy ranged between 1 day to 15 months. One patient in each arm developed retinal vein occlusion.
Perform an ophthalmological evaluation at regular intervals and any time a patient reports new or worsening visual disturbances. If serous retinopathy is diagnosed, interrupt COTELLIC until visual symptoms improve. Manage serous retinopathy with treatment interruption, dose reduction, or with treatment discontinuation [see Dosage and Administration (2.3)].
5.6 Hepatotoxicity
Hepatotoxicity can occur with COTELLIC.
The incidences of Grade 3 or 4 liver laboratory abnormalities in Trial 1 among patients receiving COTELLIC with vemurafenib compared to patients receiving vemurafenib were: 11% vs. 5% for alanine aminotransferase, 8% vs. 2.1% for aspartate aminotransferase, 1.6% vs. 1.2% for total bilirubin, and 7% vs. 3.3% for alkaline phosphatase [see Adverse Drug Reactions (6.1)]. Concurrent elevation in ALT >3 times the upper limit of normal (ULN) and bilirubin >2 × ULN in the absence of significant alkaline phosphatase >2 × ULN occurred in one patient (0.4%) receiving COTELLIC with vemurafenib and no patients receiving single-agent vemurafenib.
Monitor liver laboratory tests before initiation of COTELLIC and monthly during treatment, or more frequently as clinically indicated. Manage Grade 3 and 4 liver laboratory abnormalities with dose interruption, reduction, or discontinuation of COTELLIC [see Dosage and Administration (2.3)].
5.7 Rhabdomyolysis
Rhabdomyolysis can occur with COTELLIC.
In Trial 1, Grade 3 or 4 CPK elevations, including asymptomatic elevations over baseline, occurred in 14% of patients receiving COTELLIC with vemurafenib and 0.5% of patients receiving vemurafenib. The median time to first occurrence of Grade 3 or 4 CPK elevations was 16 days (range: 12 days to 11 months) in patients receiving COTELLIC with vemurafenib; the median time to complete resolution was 15 days (range: 9 days to 11 months). Elevation of serum CPK increase of more than 10 times the baseline value with a concurrent increase in serum creatinine of 1.5 times or greater compared to baseline occurred in 3.6% of patients receiving COTELLIC with vemurafenib and in 0.4% of patients receiving vemurafenib.
Obtain baseline serum CPK and creatinine levels prior to initiating COTELLIC, periodically during treatment, and as clinically indicated. If CPK is elevated, evaluate for signs and symptoms of rhabdomyolysis or other causes. Depending on the severity of symptoms or CPK elevation, dose interruption or discontinuation of COTELLIC may be required [see Dosage and Administration (2.3)].
5.8 Severe Photosensitivity
Photosensitivity, including severe cases, can occur with COTELLIC.
In Trial 1, photosensitivity was reported in 47% of patients receiving COTELLIC with vemurafenib: 43% of patients with Grades 1 or 2 photosensitivity and the remaining 4% with Grade 3 photosensitivity. Median time to first onset of photosensitivity of any grade was 2 months (range: 1 day to 14 months) in patients receiving COTELLIC with vemurafenib, and the median duration of photosensitivity was 3 months (range: 2 days to 14 months). Among the 47% of patients with photosensitivity reactions on COTELLIC with vemurafenib, 63% experienced resolution of photosensitivity reactions.
Advise patients to avoid sun exposure, wear protective clothing and use a broad-spectrum UVA/UVB sunscreen and lip balm (SPF ≥30) when outdoors. Manage intolerable Grade 2 or greater photosensitivity with dose modifications [see Dosage and Administration (2.3)].
5.9 Embryo-Fetal Toxicity
Based on its mechanism of action and findings from animal reproduction studies, COTELLIC can cause fetal harm when administered to a pregnant woman. In animal reproduction studies, oral administration of cobimetinib in pregnant rats during the period of organogenesis was teratogenic and embryotoxic at doses resulting in exposures [area under the curves (AUCs)] that were 0.9 to 1.4-times those observed in humans at the recommended human dose of 60 mg. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with COTELLIC, and for 2 weeks following the final dose of COTELLIC [see Use in Specific Populations (8.1, 8.3), Clinical Pharmacology (12.1)].
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the label:
- New Primary Cutaneous Malignancies [see Warnings and Precautions (5.1)]
- Hemorrhage [see Warnings and Precautions (5.2)]
- Cardiomyopathy [see Warnings and Precautions (5.3)]
- Serious Dermatologic Reactions [see Warnings and Precautions (5.4)]
- Serous Retinopathy and Retinal Vein Occlusion [see Warnings and Precautions (5.5)]
- Hepatotoxicity [see Warnings and Precautions (5.6)]
- Rhabdomyolysis [see Warnings and Precautions (5.7)]
- Severe Photosensitivity [see Warnings and Precautions (5.8)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of COTELLIC was evaluated in Trial 1, a randomized (1:1), double-blind, active-controlled trial in previously untreated patients with BRAF V600 mutation-positive, unresectable or metastatic melanoma [see Clinical Studies (14)]. All patients received vemurafenib 960 mg twice daily on Days 1–28 and received either COTELLIC 60 mg once daily (n=247) or placebo (n=246) on Days 1–21 of each 28-day treatment cycle until disease progression or unacceptable toxicity. In the COTELLIC plus vemurafenib arm, 66% percent of patients were exposed for greater than 6 months and 24% of patients were exposed for greater than 1 year. Patients with abnormal liver function tests, history of acute coronary syndrome within 6 months, evidence of Class II or greater congestive heart failure (New York Heart Association), active central nervous system lesions, or evidence of retinal pathology were excluded from Trial 1. The demographics and baseline tumor characteristics of patients enrolled in Trial 1 are summarized in Clinical Studies [see Clinical Studies (14)].
In Trial 1, 15% of patients receiving COTELLIC experienced an adverse reaction that resulted in permanent discontinuation of COTELLIC. The most common adverse reactions resulting in permanent discontinuation were liver laboratory abnormalities defined as increased aspartate aminotransferase (AST) (2.4%), increased gamma glutamyltransferase (GGT) (1.6%) and increased alanine aminotransferase (ALT) (1.6%); rash (1.6%); pyrexia (1.2%); and retinal detachment (2%). Among the 247 patients receiving COTELLIC, adverse reactions led to dose interruption or reductions in 55%. The most common reasons for dose interruptions or reductions of COTELLIC were rash (11%), diarrhea (9%), chorioretinopathy (7%), pyrexia (6%), vomiting (6%), nausea (5%), and increased creatine phosphokinase (CPK) (4.9%). The most common (≥20%) adverse reactions with COTELLIC were diarrhea, photosensitivity reaction, nausea, pyrexia, and vomiting.
Table 3. Incidence of Adverse Drug Reactions Occurring in ≥10% (All Grades) of Patients Receiving COTELLIC with Vemurafenib and at a Higher Incidence* than Patients Receiving Vemurafenib in Trial 1 Adverse reactions COTELLIC + Vemurafenib
(n=247)Placebo + Vemurafenib
(n=246)All Grades†
(%)Grades 3–4
(%)All Grades
(%)Grades 3–4
(%)- * ≥5% for All Grades or ≥2% for Grades 3–4 incidence in patients receiving COTELLIC with vemurafenib compared with patients receiving vemurafenib as a single agent
- † NCI CTCAE, v4.0.
- ‡ Includes stomatitis, aphthous stomatitis, mouth ulceration, and mucosal inflammation
- § Includes solar dermatitis, sunburn, photosensitivity reaction
- ¶ Includes hemorrhage, rectal hemorrhage, melena, hemorrhoidal hemorrhage, gastrointestinal hemorrhage, hematemesis, hematochezia, gingival bleeding, metrorrhagia, uterine hemorrhage, hemorrhagic ovarian cyst, menometrorrhagia, menorrhagia, vaginal hemorrhage, hemoptysis, pulmonary, cerebral, subarachnoid hemorrhage, subgaleal hematoma, hematuria, epistaxis, contusion, traumatic hematoma, ecchymosis, purpura, nail bed bleeding, ocular, eye, conjunctival, and retinal hemorrhage
- # Includes vision blurred, visual acuity reduced, visual impairment
- Þ Includes retinal detachment, detachment of retinal pigment epithelium, detachment of macular retinal pigment epithelium
GASTROINTESTINAL DISORDERS Diarrhea 60 6 31 1 Nausea 41 1 25 1 Vomiting 24 1 13 1 Stomatitis‡ 14 1 8 0 SKIN AND SUBCUTANEOUS TISSUE DISORDERS Photosensitivity reaction§ 46 4 35 0 Acneiform dermatitis 16 2 11 1 GENERAL DISORDERS AND ADMINISTRATION SITE CONDITIONS Pyrexia 28 2 23 0 Chills 10 0 5 0 VASCULAR DISORDERS Hypertension 15 4 8 2 Hemorrhage¶ 13 1 7 <1 EYE DISORDERS Vision impaired# 15 <1 4 0 Chorioretinopathy 13 <1 <1 0 Retinal detachmentÞ 12 2 <1 0 Adverse reactions of vemurafenib which occurred at a lower rate in patients receiving COTELLIC plus vemurafenib were alopecia (15%), hyperkeratosis (11%), and erythema (10%).
The following adverse reactions (all grades) of COTELLIC were reported with <10% incidence in Trial 1:
Respiratory, thoracic and mediastinal disorders: Pneumonitis
Table 4. Incidence of Laboratory Abnormalities Occurring in ≥10% (All Grades) or ≥2% (Grades 3–4) of Patients in Trial 1* Laboratory COTELLIC + Vemurafenib Placebo + Vemurafenib All Grades† Grades 3–4† All Grades† Grades 3–4† % % % % AST - aspartate aminotransferase, ALT - alanine aminotransferase, GGT - gamma-glutamyltransferase - * All the percentages are based on the number of patients who had a baseline result and at least one on-study laboratory test. The laboratory results are available for a total of 233~244 patients for COTELLIC, and 232~243 for vemurafenib, except where indicated.
- † NCI CTCAE v4.0.
- ‡ Increase creatine phosphokinase, n=213 for COTELLIC and 217 for vemurafenib.
- § Lymphopenia, n=185 for COTELLIC, and 181 for vemurafenib.
Chemistry Increased creatinine 99.6 3.3 99.6 0.4 Increased AST 73 8 44 2.1 Increased ALT 68 11 55 5 Increased alkaline phosphatase 71 7 56 3.3 Increased creatine phosphokinase‡ 79 14 16 0.5 Hypophosphatemia 68 12 38 6 Increased GGT 65 21 61 17 Hyponatremia 38 6 33 2.1 Hypoalbuminemia 42 0.8 20 0.4 Hypokalemia 25 4.5 17 3.3 Hyperkalemia 26 2.9 15 0.4 Hypocalcemia 24 0.4 10 1.7 Hematology Anemia 69 2.5 57 3.3 Lymphopenia§ 73 10 55 8 Thrombocytopenia 18 0 10 0 -
7 DRUG INTERACTIONS
7.1 Effect of Strong or Moderate CYP3A Inhibitors on Cobimetinib
Coadministration of COTELLIC with itraconazole (a strong CYP3A4 inhibitor) increased cobimetinib systemic exposure by 6.7-fold. Avoid concurrent use of COTELLIC and strong or moderate CYP3A inhibitors. If concurrent short term (14 days or less) use of moderate CYP3A inhibitors including certain antibiotics (e.g., erythromycin, ciprofloxacin) is unavoidable for patients who are taking COTELLIC 60 mg, reduce COTELLIC dose to 20 mg. After discontinuation of a moderate CYP3A inhibitor, resume COTELLIC at the previous dose [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)]. Use an alternative to a strong or moderate CYP3A inhibitor in patients who are taking a reduced dose of COTELLIC (40 or 20 mg daily) [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)].
7.2 Effect of Strong or Moderate CYP3A Inducers on Cobimetinib
Coadministration of COTELLIC with a strong CYP3A inducer may decrease cobimetinib systemic exposure by more than 80% and reduce its efficacy. Avoid concurrent use of COTELLIC and strong or moderate CYP3A inducers including but not limited to carbamazepine, efavirenz, phenytoin, rifampin, and St. John's Wort [see Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings from animal reproduction studies and its mechanism of action, COTELLIC can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. There are no available data on the use of COTELLIC during pregnancy. In animal reproduction studies, oral administration of cobimetinib in pregnant rats during organogenesis was teratogenic and embryotoxic at exposures (AUC) that were 0.9 to 1.4-times those observed in humans at the recommended human dose of 60 mg [see Data]. Advise pregnant women of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2–4% and 15–20%, respectively.
Animal Data
Administration of cobimetinib to pregnant rats during the period of organogenesis resulted in increased post-implantation loss, including total litter loss, at exposures (AUC) of 0.9–1.4 times those in humans at the recommended dose of 60 mg. Post-implantation loss was primarily due to early resorptions. Fetal malformations of the great vessels and skull (eye sockets) occurred at the same exposures.
8.2 Lactation
Risk Summary
There is no information regarding the presence of cobimetinib in human milk, effects on the breastfed infant, or effects on milk production. Because of the potential for serious adverse reactions in a breastfed infant, advise a nursing woman not to breastfeed during treatment with COTELLIC and for 2 weeks after the final dose.
8.3 Females and Males of Reproductive Potential
Contraception
Females
COTELLIC can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)]. Advise females of reproductive potential to use effective contraception during treatment with COTELLIC and for 2 weeks after the final dose of COTELLIC.
Infertility
Females and Males
Based on findings in animals, COTELLIC may reduce fertility in females and males of reproductive potential [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
The safety and effectiveness of COTELLIC have not been established in pediatric patients.
Juvenile Animal Data
In a 4-week juvenile rat toxicology study, daily oral doses of 3 mg/kg (approximately 0.13–0.5 times the adult human AUC at the recommended dose of 60 mg) between postnatal Days 10–17 (approximately equivalent to ages 1–2 years in humans) were associated with mortality, the cause of which was not defined.
8.5 Geriatric Use
Clinical studies of cobimetinib did not include sufficient numbers of patients aged 65 years and older to determine whether they respond differently from younger patients.
8.6 Hepatic Impairment
Adjustment in the starting dose of COTELLIC is not required in patients with mild (Child-Pugh score A), moderate (Child-Pugh B) or severe (Child-Pugh C) hepatic impairment [see Clinical Pharmacology (12.3)].
8.7 Renal Impairment
No dedicated pharmacokinetic trial in patients with renal impairment has been conducted. Dose adjustment is not recommended for mild to moderate renal impairment (CLcr 30 to 89 mL/min) based on the results of the population pharmacokinetic analysis. A recommended dose has not been established for patients with severe renal impairment [see Clinical Pharmacology (12.3)].
- 10 OVERDOSAGE
-
11 DESCRIPTION
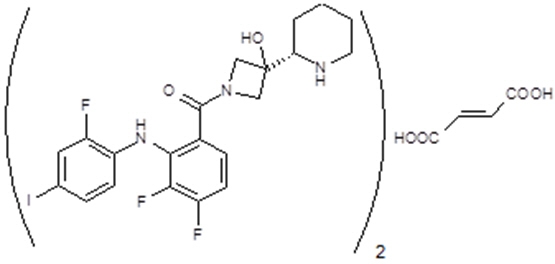
Cobimetinib fumarate is a kinase inhibitor. The chemical name is (S)-[3,4-difluoro-2-(2-fluoro-4-iodophenylamino)phenyl] [3-hydroxy-3-(piperidin-2-yl)azetidin-1-yl]methanone hemifumarate. It has a molecular formula C46H46F6I2N6O8 (2 C21H21F3IN3O2 ∙ C4H4O4) with a molecular mass of 1178.71 as a fumarate salt. Cobimetinib fumarate has the following chemical structure:
Cobimetinib is a fumarate salt appearing as white to off-white solid and exhibits a pH dependent solubility.
COTELLIC (cobimetinib) tablets are supplied as white, round, film-coated 20 mg tablets for oral administration, debossed on one side with "COB". Each 20 mg tablet contains 22 mg of cobimetinib fumarate, which corresponds to 20 mg of the cobimetinib free base.
The inactive ingredients of COTELLIC are: Tablet Core: microcrystalline cellulose, lactose monohydrate, croscarmellose sodium, magnesium stearate. Coating: polyvinyl alcohol, titanium dioxide, polyethylene glycol 3350, talc.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Cobimetinib is a reversible inhibitor of mitogen-activated protein kinase (MAPK)/extracellular signal regulated kinase 1 (MEK1) and MEK2. MEK proteins are upstream regulators of the extracellular signal-related kinase (ERK) pathway, which promotes cellular proliferation. BRAF V600E and K mutations result in constitutive activation of the BRAF pathway which includes MEK1 and MEK2. In mice implanted with tumor cell lines expressing BRAF V600E, cobimetinib inhibited tumor cell growth.
Cobimetinib and vemurafenib target two different kinases in the RAS/RAF/MEK/ERK pathway. Compared to either drug alone, coadministration of cobimetinib and vemurafenib resulted in increased apoptosis in vitro and reduced tumor growth in mouse implantation models of tumor cell lines harboring BRAF V600E mutations. Cobimetinib also prevented vemurafenib-mediated growth enhancement of a wild-type BRAF tumor cell line in an in vivo mouse implantation model.
12.2 Pharmacodynamics
Cardiac Electrophysiology
Clinically relevant QT prolongation has been reported with vemurafenib, further QTc prolongation was not observed when cobimetinib 60 mg daily was co-administered with vemurafenib. Monitor ECG and electrolytes before initiating treatment and routinely during treatment with cobimetinib, when administered with vemurafenib. Review the Full Prescribing Information for vemurafenib for details.
12.3 Pharmacokinetics
The pharmacokinetics of cobimetinib was studied in healthy subjects and cancer patients. Cobimetinib exhibits linear pharmacokinetics in the dose range of 3.5 to 100 mg (i.e., 0.06 to 1.7 times the recommended dosage). Following oral administration of COTELLIC 60 mg once daily, steady-state was reached by 9 days with a mean accumulation ratio of 2.4-fold (44% CV).
Absorption
Following oral dosing of 60 mg once daily in cancer patients, the median time to achieve peak plasma levels (Tmax) was 2.4 (range:1–24) hours, geometric mean steady-state AUC0-24h was 4340 ng∙h/mL (61% CV) and Cmax was 273 ng/mL (60% CV). The absolute bioavailability of COTELLIC was 46% (90% CI: 40%, 53%) in healthy subjects. A high-fat meal (comprised of approximately 150 calories from protein, 250 calories from carbohydrate, and 500–600 calories from fat) had no effect on cobimetinib AUC and Cmax after a single 20 mg COTELLIC was administered to healthy subjects.
Distribution
Cobimetinib is 95% bound to human plasma proteins in vitro, independent of drug concentration. No preferential binding to human red blood cells was observed (blood to plasma ratio of 0.93). The estimated apparent volume of distribution was 806 L in cancer patients based on a population PK analysis.
Elimination
Following oral administration of COTELLIC 60 mg once daily in cancer patients, the mean elimination half-life (t1/2) was 44 (range: 23–70) hours and the mean apparent clearance (CL/F) was 13.8 L/h (61% CV).
Specific Populations
Age, Sex, and Race/Ethnicity: Based on the population pharmacokinetic analysis, age (19–88 years), sex, or race/ethnicity does not have a clinically important effect on the systemic exposure of cobimetinib.
Hepatic Impairment
Following a single 10 mg COTELLIC dose, the geometric mean total cobimetinib exposure (AUCinf) values were similar in subjects with mild or moderate hepatic impairment and was decreased by 31% in subjects with severe hepatic impairment compared to subjects with normal hepatic function. [see Use in Specific Populations (8.6)].
Renal Impairment
Cobimetinib undergoes minimal renal elimination. Cobimetinib exposures were similar in 151 patients with mild renal impairment (CLcr 60 to 89 mL/min), 48 patients with moderate renal impairment (CLcr 30 to 59 mL/min) and 286 patients with normal renal function (CLcr ≥90 mL/min) [see Use in Specific Populations (8.7)].
Drug Interaction Studies
Vemurafenib: Coadministration of COTELLIC 60 mg once daily and vemurafenib 960 mg twice daily resulted in no clinically relevant pharmacokinetic drug interactions.
Effect of Strong and Moderate CYP3A Inhibitors on Cobimetinib: In vitro studies show that cobimetinib is a substrate of CYP3A. Coadministration of itraconazole (a strong CYP3A inhibitor) 200 mg once daily for 14 days with a single 10 mg cobimetinib dose increased mean cobimetinib AUC (90% CI) by 6.7-fold (5.6, 8.0) and mean Cmax (90% CI) by 3.2-fold (2.7, 3.7) in 15 healthy subjects. Simulations showed that predicted steady-state concentrations of cobimetinib at a reduced dose of 20 mg administered concurrently with short-term (less than 14 days) treatment of a moderate CYP3A inhibitor were similar to observed steady-state concentrations of cobimetinib at the 60 mg dose alone [see Drug Interactions (7.1)].
Effect of Strong and Moderate CYP3A Inducers on Cobimetinib: Based on simulations, cobimetinib exposures would decrease by 83% when coadministered with a strong CYP3A inducer and by 73% when coadministered with a moderate CYP3A inducer [see Drug Interactions (7.2)].
Effect of Cobimetinib on CYP Substrates: Coadministration of cobimetinib 60 mg once daily for 15 days with a single 30 mg dose of dextromethorphan (sensitive CYP2D6 substrate) or a single 2 mg dose of midazolam (sensitive CYP3A substrate) to 20 patients with solid tumors did not change dextromethorphan or midazolam systemic exposure. In vitro data indicated that cobimetinib may inhibit CYP3A and CYP2D6. Cobimetinib at clinically relevant concentrations is not an inhibitor of CYP1A2, 2B6, 2C8, 2C9 and 2C19 or inducer of CYP1A2, 2B6 and 3A4.
Effect of Transporters on Cobimetinib: Cobimetinib is a substrate of efflux transporter P-glycoprotein (P-gp), but is not a substrate of Breast Cancer Resistance Protein (BCRP), Organic Anion Transporting Polypeptide (OATP1B1 or OATP1B3) or Organic Cation Transporter (OCT1) in vitro. Drugs that inhibit P-gp may increase cobimetinib concentrations.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies with cobimetinib have not been conducted. Cobimetinib was not genotoxic in studies evaluating reverse mutations in bacteria, chromosomal aberrations in mammalian cells, and micronuclei in bone marrow of rats.
No dedicated fertility studies have been performed with cobimetinib in animals; however, effects on reproductive tissues observed in general toxicology studies conducted in animals suggest that there is potential for cobimetinib to impair fertility. In female rats, degenerative changes included increased apoptosis/necrosis of corpora lutea and vaginal epithelial cells at cobimetinib doses approximately twice those in humans at the clinically recommended dose of 60 mg based on body surface area. In male dogs, testicular degeneration occurred at exposures as low as approximately 0.1 times the exposure in humans at the clinically recommended dose of 60 mg.
-
14 CLINICAL STUDIES
The safety and efficacy of cobimetinib was established in a multicenter, randomized (1:1), double-blinded, placebo-controlled trial conducted in 495 patients with previously untreated, BRAF V600 mutation-positive, unresectable or metastatic, melanoma. The presence of BRAF V600 mutation was detected using the cobas® 4800 BRAF V600 mutation test. All patients received vemurafenib 960 mg orally twice daily on days 1–28 and were randomized to receive COTELLIC 60 mg or matching placebo orally once daily on days 1–21 of an every 28-day cycle. Randomization was stratified by geographic region (North America vs. Europe vs. Australia/New Zealand/others) and disease stage (unresectable Stage IIIc, M1a, or M1b vs. Stage M1c). Treatment continued until disease progression or unacceptable toxicity. Patients randomized to receive placebo were not offered COTELLIC at the time of disease progression.
The major efficacy outcome was investigator-assessed progression-free survival (PFS) per RECIST v1.1. Additional efficacy outcomes were investigator-assessed confirmed objective response rate, overall survival, PFS as assessed by blinded independent central review, and duration of response.
The median age of the study population was 55 years (range 23 to 88 years), 58% of patients were male, 93% were White and 5% had no race reported, 60% had stage M1c disease, 72% had a baseline ECOG performance status of 0, 45% had an elevated baseline serum lactate dehydrogenase (LDH), 10% had received prior adjuvant therapy, and <1% had previously treated brain metastases. Patients with available tumor samples were retrospectively tested using next generation sequencing to further classify mutations as V600E or V600K; test results were obtained on 81% of randomized patients. Of these, 86% were identified as having a V600E mutation and 14% as having a V600K mutation.
Efficacy results are summarized in Table 5 and Figure 1.
Table 5 Efficacy Results from Trial 1 COTELLIC + Vemurafenib
(n=247)Placebo + Vemurafenib
(n=248)CI - Confidence Intervals; NE - not estimable - * Based on the final overall survival analysis, conducted after 16 months from the PFS primary analysis
Progression-Free Survival (Investigator-Assessed) Number of Events (%) 143 (58%) 180 (73%) Progression 131 169 Death 12 11 Median PFS, months (95% CI) 12.3 (9.5, 13.4) 7.2 (5.6,7.5) Hazard Ratio (95% CI) 0.56 (0.45, 0.70) p-value (stratified log-rank test) <0.001 Overall Survival * Number of Deaths (%) 114 (46.2%) 141 (56.9%) Median OS, months (95% CI) 22.3
(20.3, NE)17.4
(15.0, 19.8)Hazard Ratio (95% CI) 0.69 (0.54,0.88) p -value (stratified log-rank test) 0.0032 Objective Response Rate Objective Response Rate 70% 50% (95% CI) (64%, 75%) (44%, 56%) Complete Response 16% 10% Partial Response 54% 40% p-value <0.001 Median Duration of Response, months (95% CI) 13.0 (11.1, 16.6) 9.2 (7.5, 12.8) Figure 1 Kaplan-Meier Curves of Overall Survival
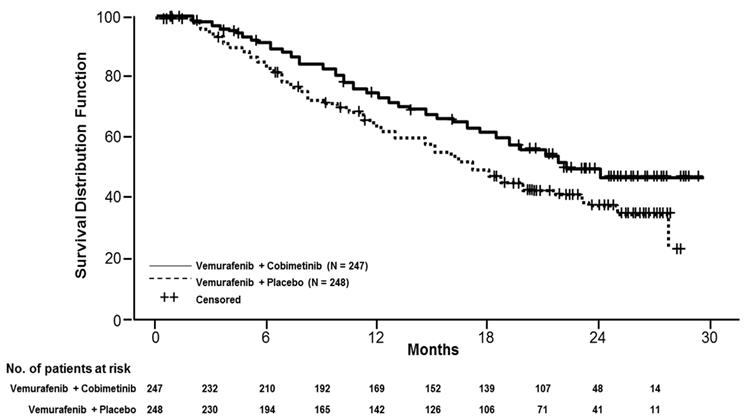
The effect on PFS was also supported by analysis of PFS based on the assessment by blinded independent review. A trend favoring the cobimetinib with vemurafenib arm was observed in exploratory subgroup analyses of PFS, OS, and ORR in both BRAF V600 mutation subtypes (V600E or V600K) in the 81% of patients in this trial where BRAF V600 mutation type was determined.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
COTELLIC (cobimetinib) is supplied as 20 mg film-coated tablets debossed on one side with "COB". COTELLIC tablets are available in bottles of 63 tablets.
NDC: 50242-717-01
-
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information).
Inform patients of the following:
New primary cutaneous malignancies: Advise patients to contact their health care provider immediately for change in or development of new skin lesions [see Warnings and Precautions (5.1)].
Hemorrhage: Instruct patients to contact their healthcare provider to seek immediate medical attention for signs or symptoms of unusual severe bleeding or hemorrhage [see Warnings and Precautions (5.2)].
Cardiomyopathy: Advise patients to report any history of cardiac disease and of the requirement for cardiac monitoring prior to and during COTELLIC administration. Instruct patients to immediately report any signs or symptoms of left ventricular dysfunction to their healthcare provider [see Warnings and Precautions (5.3)].
Serious dermatologic reactions: Instruct patients to contact their healthcare provider to immediately report severe skin changes [see Warnings and Precautions (5.4)].
Serous retinopathy and retinal vein occlusion: Instruct patients to immediately contact their healthcare provider if they experience any changes in their vision [see Warnings and Precautions (5.5)].
Hepatotoxicity: Advise patients that treatment with COTELLIC requires monitoring of their liver function. Instruct patients to report any signs or symptoms of liver dysfunction [see Warnings and Precautions (5.6)].
Rhabdomyolysis: Instruct patients to report any signs and symptoms of muscle pain or weakness to their healthcare provider [see Warnings and Precautions (5.7)].
Severe photosensitivity: Advise patients to avoid sun exposure, wear protective clothing, and use broad spectrum UVA/UVB sunscreen and lip balm (SPF ≥30) when outdoors [see Warnings and Precautions (5.8)].
Embryo-fetal toxicity: Advise females of reproductive potential of the potential risk to a fetus. Advise females to contact their healthcare provider if they become pregnant, or if pregnancy is suspected, during treatment with COTELLIC [see Warnings and Precautions (5.9), Use in Specific Populations (8.1)].
Females of reproductive potential: Advise females of reproductive potential to use effective contraception during treatment with COTELLIC and for at least 2 weeks after the final dose of COTELLIC [see Use in Specific Populations (8.3)].
Lactation: Advise females not to breastfeed during treatment with COTELLIC and for 2 weeks after the final dose [see Use in Specific Populations (8.2)].
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
COTELLIC® (co-TELL-ic)
(cobimetinib)
tabletImportant: If your healthcare provider prescribes vemurafenib, also read the Medication Guide that comes with vemurafenib. What is COTELLIC? COTELLIC is a prescription medicine that is used with the medicine vemurafenib, to treat a type of skin cancer called melanoma: - that has spread to other parts of the body or cannot be removed by surgery, and
- that has a certain type of abnormal "BRAF" gene
Your healthcare provider will perform a test to make sure that COTELLIC is right for you. It is not known if COTELLIC is safe and effective in children under 18 years of age. Before you take COTELLIC, tell your healthcare provider about all of your medical conditions, including if you: - have skin problems or history a of skin problems, other than melanoma
- have bleeding problems, any medical conditions and/or on any medications that increase your risk of bleeding
- have heart problems
- have eye problems
- have liver problems
- have muscle problems
- are pregnant or plan to become pregnant. COTELLIC can harm your unborn baby.
- Females who are able to become pregnant should use effective birth control during treatment with COTELLIC and for 2 weeks after the final dose of COTELLIC.
- Talk to your healthcare provider about birth control methods that may be right for you.
- Tell your healthcare provider right away if you become pregnant or think you are pregnant during treatment with COTELLIC.
- are breastfeeding or plan to breastfeed. It is not known if COTELLIC passes into your breast milk. Do not breastfeed during treatment with COTELLIC and for 2 weeks after the final dose. Talk to your healthcare provider about the best way to feed your baby during this time.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Certain medicines may affect the blood levels of COTELLIC. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine. How should I take COTELLIC? - Take COTELLIC exactly as your healthcare provider tells you. Do not change your dose or stop taking COTELLIC unless your healthcare provider tells you to.
- Take COTELLIC one time a day for 21 days, followed by 7 days off treatment, to complete a 28-day treatment cycle.
- Take COTELLIC with or without food.
- If you miss a dose of COTELLIC or vomit after taking your dose, take your next dose as scheduled.
What should I avoid during treatment with COTELLIC? Avoid sunlight during treatment with COTELLIC. COTELLIC can make your skin sensitive to sunlight. You may burn more easily and get severe sunburns. To help protect against sunburn: - When you go outside, wear clothes that protect your skin, including your head, face, hands, arms, and legs.
- Use lip balm and a broad-spectrum sunscreen with SPF 30 or higher.
What are the possible side effects of COTELLIC? COTELLIC may cause serious side effects, including: -
Risk of new skin cancers. COTELLIC may cause new skin cancers (cutaneous squamous cell carcinoma, keratoacanthoma, or basal cell carcinoma).
Check your skin regularly and tell your healthcare provider right away if you have any skin changes including:- new wart
- skin sore or reddish bump that bleeds or does not heal
- change in size or color of a mole
-
Bleeding problems. COTELLIC can cause serious bleeding problems.
Call your healthcare provider and get medical attention right away if you get any signs of bleeding, including:
- red or black stools (looks like tar)
- blood in your urine
- headaches
- cough up or vomit blood
- stomach (abdominal) pain
- unusual vaginal bleeding
- dizziness or weakness
- Heart problems. Your healthcare provider should do tests before and during treatment to check your heart function. Tell your healthcare provider if you get any of these signs and symptoms of heart problems:
- persistent coughing or wheezing
- shortness of breath
- swelling of your ankles and feet
- tiredness
- increased heart rate
-
Severe rash. Tell your healthcare provider right away if you get any of these symptoms:
- a rash that covers a large area of your body
- blisters
- peeling skin
- Eye problems. Tell your healthcare provider right away if you get any of these symptoms:
- blurred vision
- partly missing vision or loss of vision
- see halos
- any other vision changes
Your healthcare provider should check your eyes if you notice any of the symptoms above. - Liver problems. Your healthcare provider should do blood tests to check your liver function before and during treatment. Tell your healthcare provider right away if you get any of these symptoms:
- yellowing of your skin or the white of your eyes
- dark or brown (tea color) urine
- nausea or vomiting
- feeling tired or weak
- loss of appetite
- Muscle problems (rhabdomyolysis). COTELLIC can cause muscle problems that can be severe. Treatment with COTELLIC may increase the level of an enzyme in your blood called creatine phosphokinase (CPK) and may be a sign of muscle damage. Your healthcare provider should do a blood test to check your levels of CPK before and during treatment. Tell your healthcare provider right away if you get any of these symptoms:
- muscle aches or pain
- muscle spasms and weakness
- dark, reddish urine
- Skin Sensitivity to sunlight (photosensitivity). Skin sensitivity to sunlight during treatment with COTELLIC is common and can sometimes be severe. Tell your healthcare provider if you get any of these symptoms:
- red, painful, itchy skin that is hot to touch
- sun rash
- skin irritation
- bumps or tiny papules
- thicken, dry, wrinkled skin
See "What should I avoid during treatment with COTELLIC?" for information on protecting your skin during treatment with COTELLIC. The most common side effects of COTELLIC include: - diarrhea
- nausea
- fever
- vomiting
Your healthcare provider will take blood tests during treatment with COTELLIC. The most common changes to blood tests include: - increased blood levels of liver enzymes (GGT, ALT, or AST)
- increased blood level of enzyme from muscle (creatine phosphokinase)
- decreased blood level of phosphate, sodium or potassium
- increased blood level of liver or bone enzyme (alkaline phosphatase)
- decreased blood level of a type of white blood cell (lymphocyte)
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of COTELLIC. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. You may also report side effects to Genentech at 1-888-835-2555. How should I store COTELLIC? - Store COTELLIC at room temperature below 30°C (86°F).
- Ask your healthcare provider or pharmacist how to safely throw away (dispose of) any unused or expired COTELLIC.
Keep COTELLIC and all medicine out of the reach of children. General information about the safe and effective use of COTELLIC Medicines are sometimes prescribed for purposes other than those listed in a Patient Information Leaflet. Do not use COTELLIC for a condition for which it was not prescribed. Do not give COTELLIC to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about COTELLIC that is written for health professionals. What are the ingredients in COTELLIC? Active ingredient: cobimetinib fumarate Inactive ingredients: Tablet Core: microcrystalline cellulose, lactose monohydrate, croscarmellose sodium, magnesium stearate Coating: polyvinyl alcohol, titanium dioxide, polyethylene glycol 3350, talc Distributed by: Genentech USA, Inc., A Member of the Roche Group, 1 DNA Way, South San Francisco, CA 94080-4990. COTELLIC® is a registered trademark of Genentech, Inc. ©2016 Genentech, Inc. All rights reserved. For more information go to www.COTELLIC.com or call 1-877-436-3683. This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: May 2016 -
SPL UNCLASSIFIED SECTION
Representative sample of labeling (see the HOW SUPPLIED section for complete listing):
- PRINCIPAL DISPLAY PANEL - 63 Tablet Bottle Label
-
INGREDIENTS AND APPEARANCE
COTELLIC
cobimetinib tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 50242-717 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COBIMETINIB FUMARATE (UNII: 6EXI96H8SV) (COBIMETINIB - UNII:ER29L26N1X) COBIMETINIB 20 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) TALC (UNII: 7SEV7J4R1U) Product Characteristics Color WHITE Score no score Shape ROUND Size 7mm Flavor Imprint Code COB Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50242-717-01 63 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/10/2015 2 NDC: 50242-717-86 63 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/10/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA206192 11/10/2015 Labeler - Genentech, Inc. (080129000) Establishment Name Address ID/FEI Business Operations F. Hoffmann-La Roche Ltd 482242971 API MANUFACTURE(50242-717) , ANALYSIS(50242-717) , MANUFACTURE(50242-717) Establishment Name Address ID/FEI Business Operations F. Hoffmann-La Roche Ltd 485244961 ANALYSIS(50242-717) Establishment Name Address ID/FEI Business Operations Genentech, Inc. 080129000 ANALYSIS(50242-717)
Trademark Results [Cotellic]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 COTELLIC 86305545 4914506 Live/Registered |
GENENTECH, INC. 2014-06-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
