CLINDAMYCIN PHOSPHATE AND TRETINOIN gel
Clindamycin Phosphate and Tretinoin by
Drug Labeling and Warnings
Clindamycin Phosphate and Tretinoin by is a Prescription medication manufactured, distributed, or labeled by Solaris Pharma Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CLINDAMYCIN PHOSPHATE AND TRETINOIN GEL safely and effectively. See full prescribing information for CLINDAMYCIN PHOSPHATE AND TRETINOIN GEL.
CLINDAMYCIN PHOSPAHTE AND TRETINOIN gel, for topical use only
Initial U.S. Approval: 2006
INDICATIONS AND USAGE
- Clindamycin phosphate and tretinoin gel is a lincosamide antibiotic and retinoid combination product indicated for the topical treatment of acne vulgaris in patients 12 years and older. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
- Topical gel: clindamycin phosphate 1.2% and tretinoin 0.025% in 30-gram and 60-gram tubes. (3)
CONTRAINDICATIONS
- Clindamycin phosphate and tretinoin gel is contraindicated in patients with regional enteritis, ulcerative colitis, or history of antibiotic-associated colitis. (4)
WARNINGS AND PRECAUTIONS
- Colitis: Clindamycin can cause severe colitis, which may result in death. Diarrhea, bloody diarrhea, and colitis (including pseudomembranous colitis) have been reported with the use of clindamycin. Clindamycin phosphate and tretinoin gel should be discontinued if significant diarrhea occurs. (5.1)
- Ultraviolet Light and Environmental Exposure: Avoid exposure to sunlight, sunlamps, and weather extremes. Wear sunscreen daily. (5.2)
ADVERSE REACTIONS
- Observed local treatment-related adverse reactions (≥ 1%) in clinical studies with clindamycin phosphate and tretinoin gel were application site reactions, including dryness, irritation, exfoliation, erythema, pruritus, and dermatitis. Sunburn was also reported. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Solaris Pharma Corporation at 1-833-919-0527 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Clindamycin phosphate and tretinoin gel should not be used in combination with erythromycin-containing products because of its clindamycin component. (7.1)
USE IN SPECIFIC POPULATIONS
- Pediatric Use: The efficacy and safety have not been established in pediatric patients below the age of 12 years. (8.4)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 1/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Colitis
5.2 Ultraviolet Light and Environmental Exposure
6 ADVERSE REACTIONS
6.1 Adverse Reactions in Clinical Studies
7 DRUG INTERACTIONS
7.1 Erythromycin
7.2 Neuromuscular Blocking Agents
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
Instructions for Use
Skin Irritation
Colitis
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
Clindamycin phosphate and tretinoin gel should be applied once daily in the evening, gently rubbing the medication to lightly cover the entire affected area. Approximately a pea-sized amount will be needed for each application. Avoid the eyes, lips, and mucous membranes.
Clindamycin phosphate and tretinoin gel is not for oral, ophthalmic, or intravaginal use.
-
3 DOSAGE FORMS AND STRENGTHS
Clindamycin phosphate and tretinoin gel, 1.2%/0.025%, containing clindamycin phosphate 1.2% and tretinoin 0.025%, is a yellow, opaque topical gel. Each gram of clindamycin phosphate and tretinoin gel, 1.2%/0.025% contains, as dispensed, 10 mg (1%) clindamycin as clindamycin phosphate, and 0.25 mg (0.025%) tretinoin solubilized in an aqueous-based gel.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Colitis
Systemic absorption of clindamycin has been demonstrated following topical use. Diarrhea, bloody diarrhea, and colitis (including pseudomembranous colitis) have been reported with the use of topical clindamycin. If significant diarrhea occurs, clindamycin phosphate and tretinoin gel should be discontinued.
Severe colitis has occurred following oral or parenteral administration of clindamycin with an onset of up to several weeks following cessation of therapy. Antiperistaltic agents such as opiates and diphenoxylate with atropine may prolong and/or worsen severe colitis. Severe colitis may result in death.
Studies indicate a toxin(s) produced by clostridia is one primary cause of antibiotic-associated colitis. The colitis is usually characterized by severe persistent diarrhea and severe abdominal cramps and may be associated with the passage of blood and mucus. Stool cultures for Clostridium difficile and stool assay for C. difficile toxin may be helpful diagnostically.
5.2 Ultraviolet Light and Environmental Exposure
Exposure to sunlight, including sunlamps, should be avoided during the use of clindamycin phosphate and tretinoin gel. Patients with sunburn should be advised not to use the product until fully recovered because of heightened susceptibility to sunlight as a result of the use of tretinoin. Patients who may be required to have considerable sun exposure due to occupation and those with inherent sensitivity to the sun should exercise particular caution. Daily use of sunscreen products and protective apparel (e.g., a hat) are recommended. Weather extremes, such as wind or cold, also may be irritating to patients under treatment with clindamycin phosphate and tretinoin gel.
-
6 ADVERSE REACTIONS
6.1 Adverse Reactions in Clinical Studies
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in clinical practice.
The safety data reflect exposure to clindamycin phosphate and tretinoin gel in 1,104 patients with acne vulgaris. Patients were 12 years or older and were treated once daily in the evening for 12 weeks. Adverse reactions that were reported in ≥1% of patients treated with clindamycin phosphate and tretinoin gel are presented in Table 1.
Table 1: Treatment-Related Adverse Reactions Reported by ≥1% of Patients Clindamycin Phosphate
and Tretinoin Gel
N = 1,104
n (%)
Clindamycin Gel
N = 1,091
n (%)Tretinoin
Gel
N = 1,084
n (%)Vehicle
Gel
N = 552
n (%)Patients with at least one adverse reaction 140 (13) 38 (3) 141 (13) 17 (3) Application site dryness 64 (6) 12 (1) 62 (6) 3 (1) Application site irritation 50 (5) 4 (<1) 57 (5) 5 (1) Application site exfoliation 50 (5) 2 (<1) 56 (5) 2 (<1) Application site erythema 40 (4) 6 (1) 39 (4) 3 (1) Application site pruritus 26 (2) 7 (1) 23 (2) 6 (1) Sunburn 11 (1) 6 (1) 7 (1) 3 (1) Application site dermatitis 6 (1) 0 (0) 8 (1) 1 (<1) Local skin reactions actively assessed at baseline and end of treatment with a score >0 are presented in Table 2.
Table 2: Clindamycin Phosphate and Tretinoin Gel - Treated Patients with Local Skin Reactions Clindamycin Phosphate
and
Tretinoin Gel
Vehicle Gel Local Reaction Baseline
N = 476
(%)End of Treatment
N = 409
(%)Baseline
N = 219
(%)End of Treatment
N = 209
(%)Erythema 24% 21% 31% 35% Scaling 8% 19% 14% 12% Dryness 11% 22% 18% 13% Burning 8% 13% 8% 4% Itching 17% 15% 22% 14% During the 12 weeks of treatment, each local skin reaction peaked at Week 2 and gradually reduced thereafter.
-
7 DRUG INTERACTIONS
7.1 Erythromycin
Clindamycin phosphate and tretinoin gel should not be used in combination with erythromycin-containing products due to possible antagonism to the clindamycin component. In vitro studies have shown antagonism between these 2 antimicrobials. The clinical significance of this in vitro antagonism is not known.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C.
There are no well-controlled studies in pregnant women treated with clindamycin phosphate and tretinoin gel. Clindamycin phosphate and tretinoin gel should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. A limit teratology study performed in Sprague Dawley rats treated topically with clindamycin phosphate and tretinoin gel or 0.025% tretinoin gel at a dose of 2 mL/kg during gestation days 6 to 15 did not result in teratogenic effects. Although no systemic levels of tretinoin were detected, craniofacial and heart abnormalities were described in drug-treated groups. These abnormalities are consistent with retinoid effects and occurred at 16 times the recommended clinical dose assuming 100% absorption and based on body surface area comparison. For purposes of comparison of the animal exposure to human exposure, the recommended clinical dose is defined as 1 g of clindamycin phosphate and tretinoin gel applied daily to a 50-kg person.
Clindamycin: Reproductive developmental toxicity studies performed in rats and mice using oral doses of clindamycin up to 600 mg/kg/day (480 and 240 times the recommended clinical dose based on body surface area comparison, respectively) or subcutaneous doses of clindamycin up to 180 mg/kg/day (140 and 70 times the recommended clinical dose based on body surface area comparison, respectively) revealed no evidence of teratogenicity.
Tretinoin: Oral tretinoin has been shown to be teratogenic in mice, rats, hamsters, rabbits, and primates. It was teratogenic and fetotoxic in Wistar rats when given orally at doses greater than 1 mg/kg/day (32 times the recommended clinical dose based on body surface area comparison). However, variations in teratogenic doses among various strains of rats have been reported. In the cynomologous monkey, a species in which tretinoin metabolism is closer to humans than in other species examined, fetal malformations were reported at oral doses of 10 mg/kg/day or greater, but none were observed at 5 mg/kg/day (324 times the recommended clinical dose based on body surface area comparison), although increased skeletal variations were observed at all doses. Dose-related teratogenic effects and increased abortion rates were reported in pigtail macaques.
With widespread use of any drug, a small number of birth defect reports associated temporally with the administration of the drug would be expected by chance alone. Thirty cases of temporally associated congenital malformations have been reported during 2 decades of clinical use of another formulation of topical tretinoin. Although no definite pattern of teratogenicity and no causal association have been established from these cases, 5 of the reports describe the rare birth defect category, holoprosencephaly (defects associated with incomplete midline development of the forebrain). The significance of these spontaneous reports in terms of risk to fetus is not known.
8.3 Nursing Mothers
It is not known whether clindamycin is excreted in human milk following use of clindamycin phosphate and tretinoin gel. However, orally and parenterally administered clindamycin has been reported to appear in breast milk. Because of the potential for serious adverse reactions in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. It is not known whether tretinoin is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when clindamycin phosphate and tretinoin gel is administered to a nursing woman.
8.4 Pediatric Use
Safety and effectiveness of clindamycin phosphate and tretinoin gel in pediatric patients below the age of 12 years have not been established.
Clinical trials of clindamycin phosphate and tretinoin gel included 2,086 patients aged 12 - 17 years of age with acne vulgaris. [See Clinical Studies (14).]
-
11 DESCRIPTION
Clindamycin phosphate and tretinoin gel, 1.2%/0.025%, is a fixed combination of 2 solubilized active ingredients in an aqueous-based gel. Clindamycin phosphate is a water soluble ester of the semi-synthetic antibiotic produced by a 7(S)-chloro-substitution of the 7(R)-hydroxyl group of the parent antibiotic lincomycin.
The chemical name for clindamycin phosphate is methyl 7-chloro-6,7,8-trideoxy-6-(1-methyl-trans-4-propyl-L-2-pyrrolidinecarboxamido)-1-thio-L-threo-α-D-galacto-octopyranoside 2-(dihydrogen phosphate). The structural formula for clindamycin phosphate is represented below:
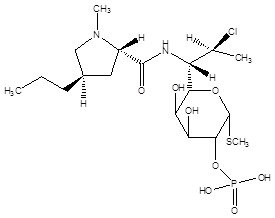
Molecular Formula: C18H34ClN2O8PS
Molecular Weight: 504.97
The chemical name for tretinoin is all-trans 3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4,6,8-nonatetraenoic acid. It is a member of the retinoid family of compounds.
The structural formula for tretinoin is represented below:
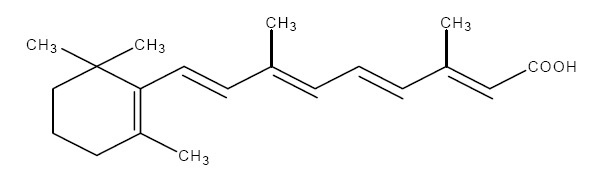
Molecular Formula: C20H28O2
Molecular Weight: 300.44
Clindamycin phosphate and tretinoin gel contains the following inactive ingredients: anhydrous citric acid, butylated hydroxytoluene, carbomer homopolymer (type C), edetate disodium, laureth 4, methylparaben, propylene glycol, purified water, and tromethamine.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Clindamycin:[See Microbiology (12.4).]
Tretinoin: Although the exact mode of action of tretinoin is unknown, current evidence suggests that topical tretinoin decreases cohesiveness of follicular epithelial cells with decreased microcomedone formation. Additionally, tretinoin stimulates mitotic activity and increased turnover of follicular epithelial cells causing extrusion of the comedones.12.3 Pharmacokinetics
In an open-label study of 17 patients with moderate-to-severe acne vulgaris, topical administration of approximately 3 grams of clindamycin phosphate and tretinoin gel once daily for 5 days, clindamycin concentrations were quantifiable in all 17 patients starting from 1 hour post-dose. All plasma clindamycin concentrations were ≤5.56 ng/mL on Day 5, with the exception of 1 subject who had a maximum clindamycin concentration of 8.73 ng/mL at 4 hours post-dose. There was no appreciable increase in systemic exposure to tretinoin, as compared with the baseline value. The average tretinoin concentration across all sampling times on Day 5 ranged from 1.19 to 1.23 ng/mL compared with the corresponding baseline mean tretinoin concentration range of 1.16 to 1.30 ng/mL.
12.4 Microbiology
No microbiology studies were conducted in the clinical trials with this product.
Mechanism of Action: Clindamycin binds to the 50S ribosomal subunit of susceptible bacteria and prevents elongation of peptide chains by interfering with peptidyl transfer, thereby suppressing protein synthesis. Clindamycin has been shown to have in vitro activity against Propionibacterium acnes (P. acnes), an organism that has been associated with acne vulgaris; however, the clinical significance of this activity against P. acnes was not examined in clinical studies with clindamycin phosphate and tretinoin gel. P. acnes resistance to clindamycin has been documented.
Inducible Clindamycin Resistance: The treatment of acne with antimicrobials is associated with the development of antimicrobial resistance in P. acnes as well as other bacteria (e.g., Staphylococcus aureus, Streptococcus pyogenes). The use of clindamycin may result in developing inducible resistance in these organisms. This resistance is not detected by routine susceptibility testing.
Cross Resistance: Resistance to clindamycin is often associated with resistance to erythromycin. -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been performed to evaluate the carcinogenic potential of clindamycin phosphate and tretinoin gel or the effect of clindamycin phosphate and tretinoin gel on fertility. Clindamycin phosphate and tretinoin gel was negative for mutagenic potential when evaluated in an in vitro Ames Salmonella reversion assay. Clindamycin phosphate and tretinoin gel was equivocal for clastogenic potential in the absence of metabolic activation when tested in an in vitro chromosomal aberration assay.
Clindamycin: Once-daily dermal administration of 1% clindamycin as clindamycin phosphate in the gel vehicle (32 mg/kg/day, 13 times the recommended clinical dose based on body surface area comparison) to mice for up to 2 years did not produce evidence of tumorigenicity.
Fertility studies in rats treated orally with up to 300 mg/kg/day of clindamycin (240 times the recommended clinical dose based on body surface area comparison) revealed no effects on fertility or mating ability.
Tretinoin: In 2 independent mouse studies where tretinoin was administered topically (0.025% or 0.1%) 3 times per week for up to 2 years no carcinogenicity was observed, with maximum effects of dermal amyloidosis. However, in a dermal carcinogenicity study in mice, tretinoin applied at a dose of 5.1 mcg (1.4 times the recommended clinical dose based on body surface area comparison) 3 times per week for 20 weeks acted as a weak promoter of skin tumor formation following a single application of dimethylbenz[α]anthracene (DMBA).
In a study in female SENCAR mice, papillomas were induced by topical exposure to DMBA followed by promotion with 12-O-tetradecanoylphorbol-13-acetate or mezerein for up to 20 weeks. Topical application of tretinoin prior to each application of promoting agent resulted in a reduction in the number of papillomas per mouse. However, papillomas resistant to topical tretinoin suppression were at higher risk for pre-malignant progression.
Tretinoin has been shown to enhance photoco-carcinogenicity in properly performed specific studies, employing concurrent or intercurrent exposure to tretinoin and UV radiation. The photoco-carcinogenic potential of the clindamycin tretinoin combination is unknown. Although the significance of these studies to humans is not clear, patients should avoid exposure to sun.
The genotoxic potential of tretinoin was evaluated in an in vitro Ames Salmonella reversion test and an in vitro chromosomal aberration assay in Chinese hamster ovary cells. Both tests were negative.
In oral fertility studies in rats treated with tretinoin, the no-observed-effect-level was 2 mg/kg/day (64 times the recommended clinical dose based on body surface area comparison).
-
14 CLINICAL STUDIES
The safety and efficacy of clindamycin phosphate and tretinoin gel, applied once daily for the treatment of acne vulgaris, was evaluated in 12-week multi-center, randomized, blinded studies in subjects 12 years and older.
Treatment response was defined as the percent of subjects who had a 2-grade improvement from baseline to Week 12 based on the Investigator’s Global Assessment (IGA) and a mean absolute change from baseline to Week 12 in 2 out of 3 (total, inflammatory and non-inflammatory) lesion counts. The IGA scoring scale used in all the clinical trials for clindamycin phosphate and tretinoin gel is as follows:
0 Clear Normal, clear skin with no evidence of acne vulgaris. 1 Almost
Clear
Skin almost clear; rare non-inflammatory lesions present, with rare non-inflamed papules (papules must be resolving and may be
hyperpigmented, though not pink-red) requiring no further treatment in the Investigator’s opinion.
2 Mild Some non-inflammatory lesions are present, with few inflammatory lesions (papules/pustules only, no nodulo-cystic lesions). 3 Moderate Non-inflammatory lesions predominate, with multiple inflammatory lesions evident; several-to-many comedones and
papules/pustules, and there may or may not be 1 small nodulo-cystic lesion.
4 Severe Inflammatory lesions are more apparent; many comedones and papules/pustules, there may or may not be a few nodulo-cystic
lesions.
5 Very Severe Highly inflammatory lesions predominate; variable numbers of comedones, many papules/pustules and nodulo-cystic lesions. In Study 1, 1,649 subjects were randomized to clindamycin phosphate and tretinoin gel, clindamycin gel, tretinoin gel, and vehicle gel. The median age of subjects was 17 years and 58% were females. At baseline, subjects had an average of 71 total lesions of which the mean number of inflammatory lesions was 25.5 lesions and the mean number of non-inflammatory lesions was 45.1 lesions. The majority of subjects enrolled with a baseline IGA score of 3. The efficacy results at Week 12 are presented in Table 3.
Table 3: Efficacy Results at Week 12
Study 1
Clindamycin Phosphate
and Tretinoin Gel
N = 476
Clindamycin
Gel
N = 467Tretinoin
Gel
N = 464Vehicle
Gel
N = 242Investigator’s Global Assessment Percentage of subjects achieving 2-Grade Improvement 36.3% 26.6% 26.1% 20.2% Percentage of subjects achieving an IGA of 0 or 1 with a 2-Grade Improvement 33.2% 24.0% 22.6% 17.8% Inflammatory Lesions:
Mean absolute reduction
Mean percentage (%) reduction
15.5
60.4%
14.5
56.5%
13.9
54.5%
11.1
43.3%
Non-inflammatory Lesions:
Mean absolute reduction
Mean percentage (%) reduction
23.2
51.0%
19.5
42.9%
22.1
47.3%
17.0
36.0%
Total Lesions:
Mean absolute reduction
Mean percentage (%) reduction
38.7
55.0%
34.0
49.0%
36.0
50.5%
28.1
39.1%
The safety and efficacy of clindamycin-tretinoin gel was also evaluated in two additional 12-week, multi-centered, randomized, blinded, studies in patients 12 years and older. A total of 2,219 subjects with mild-to-moderate acne vulgaris were treated once daily for 12 weeks. Of the 2,219 subjects, 634 subjects were treated with clindamycin-tretinoin gel. These studies demonstrated consistent outcomes.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
Clindamycin phosphate and tretinoin gel, 1.2%/0.025% is supplied as follows:
- 30 g aluminum tubes NDC: 73473-306-30
- 60 g aluminum tubes NDC: 73473-306-60
Storage and Handling
- Store at 25°C (77°F); excursions permitted from 15°C to 30°C (59°F to 86°F).
- Protect from heat.
- Protect from light.
- Protect from freezing.
- Keep out of reach of children.
- Keep tube tightly closed.
-
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information).
Instructions for Use
- At bedtime, the face should be gently washed with a mild soap and water. After patting the skin dry, apply clindamycin phosphate and tretinoin gel as a thin layer over the entire affected area (excluding the eyes and lips).
- Patients should be advised not to use more than a pea-sized amount to cover the face and not to apply more often than once daily (at bedtime) as this will not make for faster results and may increase irritation.
- A sunscreen should be applied every morning and reapplied over the course of the day as needed. Patients should be advised to avoid exposure to sunlight, sunlamp, ultraviolet light, and other medicines that may increase sensitivity to sunlight.
- Other topical products with a strong drying effect such as abrasive soaps or cleansers may cause an increase in skin irritation with clindamycin phosphate and tretinoin gel.
Skin Irritation
Clindamycin phosphate and tretinoin gel may cause irritation such as erythema, scaling, itching, burning, or stinging.
Colitis
In the event a patient treated with clindamycin phosphate and tretinoin gel experiences severe diarrhea or gastrointestinal discomfort, clindamycin phosphate and tretinoin gel should be discontinued and a physician should be contacted.
Manufactured for:
Solaris Pharma Corporation
Bridgewater, NJ 08807 -
PATIENT INFORMATION
Clindamycin Phosphate and Tretinoin Gel
Read the Patient Information that comes with clindamycin phosphate and tretinoin gel before you start using it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your doctor about your medical condition or your treatment.
What is clindamycin phosphate and tretinoin gel?
Clindamycin phosphate and tretinoin gel is prescription medicine used on the skin to treat acne in people 12 years and older.
It is not known if clindamycin phosphate and tretinoin gel is safe and effective in children under 12 years of age.
Who should not use clindamycin phosphate and tretinoin gel?
Do not use clindamycin phosphate and tretinoin gel if you have:
- Crohn’s disease
- ulcerative colitis
- had inflammation of the colon (colitis) with past antibiotic use
Talk to your doctor if you are not sure if you have one of these conditions.
What should I tell my doctor before using clindamycin phosphate and tretinoin gel?
Before using clindamycin phosphate and tretinoin gel, tell your doctor if you:
- have any allergies
- plan to have surgery with general anesthesia. One of the medicines in clindamycin phosphate and tretinoin gel can affect how certain anesthesia medicines work.
- have any other medical conditions
- are pregnant or plan to become pregnant. It is not known if clindamycin phosphate and tretinoin gel may harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if clindamycin phosphate and tretinoin gel passes into your breast milk. One of the medicines in clindamycin phosphate and tretinoin gel contains clindamycin. When clindamycin is taken by mouth or injection, it may pass into breast milk. You and your doctor should decide if you will take clindamycin phosphate and tretinoin gel or breastfeed. You should not do both.
Tell your doctor about all the medicines and skin products you use. Especially tell your doctor if you take medicine that contains erythromycin. Clindamycin phosphate and tretinoin gel should not be used with products that contain erythromycin.
Know the medicines you take. Keep a list of your medicines and show it to your doctor and pharmacist when you get a new medicine.
How should I use clindamycin phosphate and tretinoin gel?
- Use clindamycin phosphate and tretinoin gel exactly as prescribed.
- Your doctor will tell you how long to use clindamycin phosphate and tretinoin gel.
- Do not apply clindamycin phosphate and tretinoin gel more than one time each day.
- Do not use too much clindamycin phosphate and tretinoin gel, because it may irritate your skin.
Instructions for applying clindamycin phosphate and tretinoin gel:
- At bedtime, wash your face gently with a mild soap; rinse with water.
- Pat the skin dry.
- Squeeze a pea-sized amount of medication onto one fingertip. Then, gently rub over the entire affected area. Do not get clindamycin phosphate and tretinoin gel in your eyes, mouth, or on your lips.
What should I avoid while using clindamycin phosphate and tretinoin gel?
- Limit your time in sunlight. Avoid using tanning beds or sun lamps. If you have to be in sunlight, wear a wide-brimmed hat or other protective clothing. Apply a sunscreen every morning and reapply during the day as needed.
- Avoid wind and cold weather during treatment with clindamycin phosphate and tretinoin gel. These may be irritating to your skin.
- Avoid using abrasive soaps and cleansers. These may cause increased skin irritation with clindamycin phosphate and tretinoin gel.
What are the possible side effects of clindamycin phosphate and tretinoin gel?
Clindamycin phosphate and tretinoin gel may cause serious side effects, including:
- Inflammation of the colon (colitis). Clindamycin, one of the ingredients in clindamycin phosphate and tretinoin gel, can cause severe colitis that may lead to death. Stop taking clindamycin phosphate and tretinoin gel and call your doctor if you develop severe watery diarrhea, or bloody diarrhea.
- Sunburn. Clindamycin phosphate and tretinoin gel may cause your skin to become sunburned more easily. If your face is sunburned, do not use clindamycin phosphate and tretinoin gel until your sunburn is completely healed. Tretinoin, one of the medicines in clindamycin phosphate and tretinoin gel, makes your skin more sensitive to sunlight. See “What should I avoid while using clindamycin phosphate and tretinoin gel?”
Common side effects of clindamycin phosphate and tretinoin gel include:
- Skin irritation. Clindamycin phosphate and tretinoin gel may cause skin irritation such as dryness, peeling, burning, or itching.
Talk to your doctor about any side effect that bothers you or that does not go away.
These are not all the side effects with clindamycin phosphate and tretinoin gel. Ask your doctor or pharmacist for more information.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store clindamycin phosphate and tretinoin gel?
- Store clindamycin phosphate and tretinoin gel at room temperature, between 59°F to 86°F (15°C to 30°C).
- Protect from freezing.
- Keep clindamycin phosphate and tretinoin gel away from heat and light.
- Keep clindamycin phosphate and tretinoin gel and all medicines out of the reach of children.
General information about clindamycin phosphate and tretinoin gel
Medicines are sometimes prescribed for purposes other than those listed in the patient information leaflet. Do not use clindamycin phosphate and tretinoin gel for a condition for which it was not prescribed. Do not give clindamycin phosphate and tretinoin gel to other people, even if they have the same symptoms you have. It may harm them.
This patient information leaflet summarizes the most important information about clindamycin phosphate and tretinoin gel. If you would like more information, talk with your doctor. You can also ask your pharmacist or doctor for information about clindamycin phosphate and tretinoin gel that is written for healthcare professionals. For more information call 1-833-919-0527.
What are the ingredients in clindamycin phosphate and tretinoin gel?
Active Ingredients: clindamycin phosphate and tretinoin
Inactive ingredients: anhydrous citric acid, butylated hydroxytoluene, carbomer homopolymer (type C), edetate disodium, laureth 4, methylparaben, propylene glycol, purified water, and tromethamine.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Manufactured for:
Solaris Pharma Corporation
Bridgewater, NJ 08807Rev: 02/2022
-
PACKAGE LABEL PRINCIPAL DISPLAY PANEL
60 g Carton
NDC: 73473-306-60
Clindamycin Phosphate and Tretinoin Gel, 1.2%/0.025%
For Topical Use Only
Rx Only60 gram

-
INGREDIENTS AND APPEARANCE
CLINDAMYCIN PHOSPHATE AND TRETINOIN
clindamycin phosphate and tretinoin gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 73473-306 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CLINDAMYCIN PHOSPHATE (UNII: EH6D7113I8) (CLINDAMYCIN - UNII:3U02EL437C) CLINDAMYCIN 10 mg in 1 g TRETINOIN (UNII: 5688UTC01R) (TRETINOIN - UNII:5688UTC01R) TRETINOIN 0.25 mg in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73473-306-30 1 in 1 CARTON 01/03/2025 1 30 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC: 73473-306-60 1 in 1 CARTON 01/03/2025 2 60 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA212845 01/03/2025 Labeler - Solaris Pharma Corporation (079904672)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.