Lucy Belle 011-01 2nd submittal
LAURA GELLER BETTER THAN BARE TINTED MOISTURIZER BROAD SPECTRUM SUNSCREEN SPF 50 Plus Sand 400 by
Drug Labeling and Warnings
LAURA GELLER BETTER THAN BARE TINTED MOISTURIZER BROAD SPECTRUM SUNSCREEN SPF 50 Plus Sand 400 by is a Otc medication manufactured, distributed, or labeled by LUCY BELLE BIOLOGICAL TECHNOLOGY CO., LTD.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
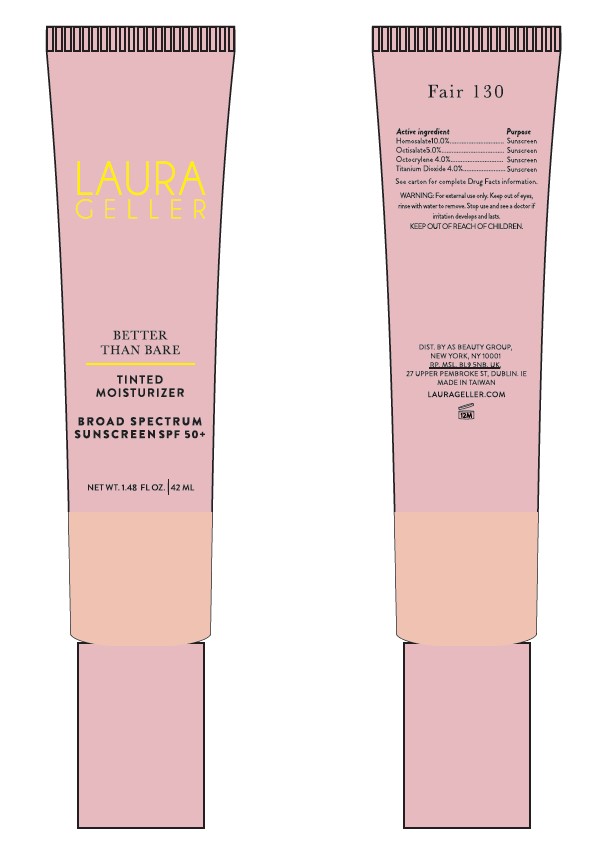
LAURA GELLER BETTER THAN BARE TINTED MOISTURIZER BROAD SPECTRUM SUNSCREEN SPF 50 PLUS SAND 400- homosalate, octisalate, octocrylene, titanium dioxide cream
LUCY BELLE BIOLOGICAL TECHNOLOGY CO., LTD.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Lucy Belle 011-01 2nd submittal
Active Ingredient(s)
Homosalate 10.0% ........... Sunscreen
Octisalate 5.0% .................. Sunscreen
Octocrylene 4.0% .............. Sunscreen
Titanium Dioxide 4.0% ....... Sunscreen
Use
Helps prevent sunburn; if used as directed with other Sun Protection Measures (see Directions), decreases the risk of skin cancer and early aging caused by the sun.
When Using This Product keep out of eyes, ears and mouth. In case of contact with eyes, rinse thoroughly with water.
Stop Use and Ask a Doctor if irritation or rash occurs. These may be signs of a serious condition.
Keep Out of Reach of Children. If swallowed, get medical help or contact the Poison Control Center Immediately.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Apply liberally 15 minutes before sun exposure. Reapply at least every 2 hours. Use a Water Resistant Sunscreen if Swimming or Sweating.
Children under 6 years of age should be supervised when using.
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer, and early skin aging. To decrease this risk regularly use a sunscreen with a broad spectrum value of 15 or higher, and other sun protection measures including: limiting time in the sun especially from 10 a.m. to 2 p.m.. Wear long sleeved shirts, pants, hats, and sunglasses.
children under 6 months: ask a doctor.
Inactive ingredients
Water (Aqua), Butylene Glycol, Polymethyl Methacrylate, Cyclopentasiloxane, Octyldodecanol, Octyldodecyl Xyloside, PEG-30 Dipolyhydroxystearate, Isononyl Isononanoate, Propanediol, Caprylic/Capric Triglyceride, Stearalkonium Hectorite, Propylene Carbonate, Cetyl PEG/PPG-10/1 Dimethicone, Lecithin, Polyhydroxystearic Acid, Ethylhexyl Palmitate, Isopropyl Myristate, Isostearic Acid, Polyglyceryl-3 Polyricinoleate, Dimethicone/Vinyl Dimethicone Crosspolymer, Silica, Dimethicone, Sodium Chloride, Triethoxycaprylylsilane, Styrene/butadiene copolymer, Phenoxyethanol, Caprylyl Glycol, Hydrogenated Coco-Glycerides, Alteromonas Ferment Extract, Helianthus Annuus (Sunflower) Seed Oil, Rosa Canina Fruit Oil, Tocopherol, Rosmarinus Officinalis (Rosemary) Extract, Vitis Vinifera (Grape) Seed Oil, Panthenol, Niacinamide, Cucumis Sativus (Cucumber) Fruit Extract. May Contain: Iron Oxides.
| LAURA GELLER BETTER THAN BARE TINTED MOISTURIZER BROAD SPECTRUM SUNSCREEN SPF 50 PLUS SAND 400
homosalate, octisalate, octocrylene, titanium dioxide cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - LUCY BELLE BIOLOGICAL TECHNOLOGY CO., LTD. (658526004) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LUCY BELLE BIOLOGICAL TECHNOLOGY CO., LTD. | 658526004 | manufacture(81536-011) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.

 42 mL NDC:
42 mL NDC: