Sentriva-ES by Singular Dreamer, LTD dba True Marker
Sentriva-ES by
Drug Labeling and Warnings
Sentriva-ES by is a Otc medication manufactured, distributed, or labeled by Singular Dreamer, LTD dba True Marker. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
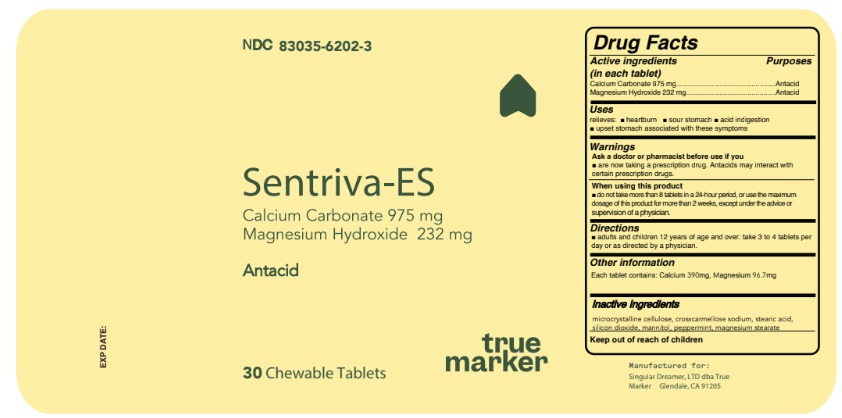
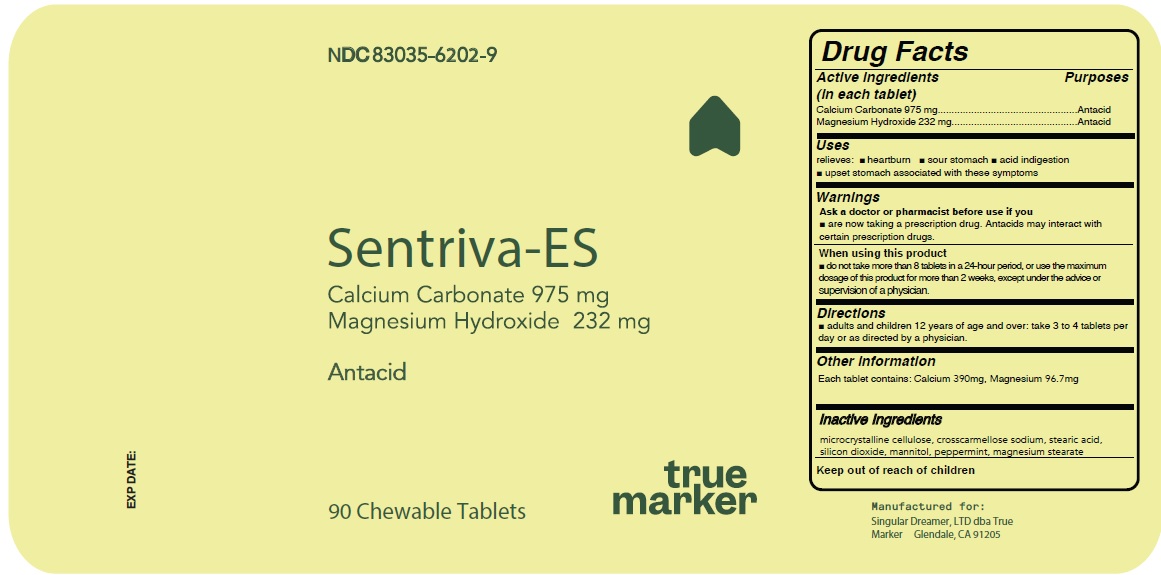
SENTRIVA-ES- calcium carbonate,magnesium hydroxide tablet, chewable
Singular Dreamer, LTD dba True Marker
----------
Active Ingredient Purposes
Calcium Carbonate USP 975 mg..................................................Antacid
Magnesium Hydroxide USP 232 mg.............................................Antacid
Warnings
Ask a doctor or pharmacist before use if you
- are now taking a prescription drug. Antacids may interact with certain prescription drugs.
When using this product
- do not take more than 8 tablets in a 24-hour period, or use the maximum dosage of this product for more than 2 weeks, except under the advice or supervision of a physician.
Directions
- adults and children 12 years of age and over: take 3 to 4 tablets per day or as directed by a physician.
| SENTRIVA-ES
calcium carbonate,magnesium hydroxide tablet, chewable |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Singular Dreamer, LTD dba True Marker (129504103) |
| Registrant - Singular Dreamer, LTD dba True Marker (129504103) |
Revised: 10/2024
Document Id: 2516b83a-292e-de62-e063-6394a90a0f05
Set id: c3d9495b-4303-4c5c-95cc-05261897a130
Version: 5
Effective Time: 20241022