DZA Brands, LLC Famotidine Tablets, 20mg Drug Facts
Healthy Accents Famotidine by
Drug Labeling and Warnings
Healthy Accents Famotidine by is a Otc medication manufactured, distributed, or labeled by DZA Brands LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
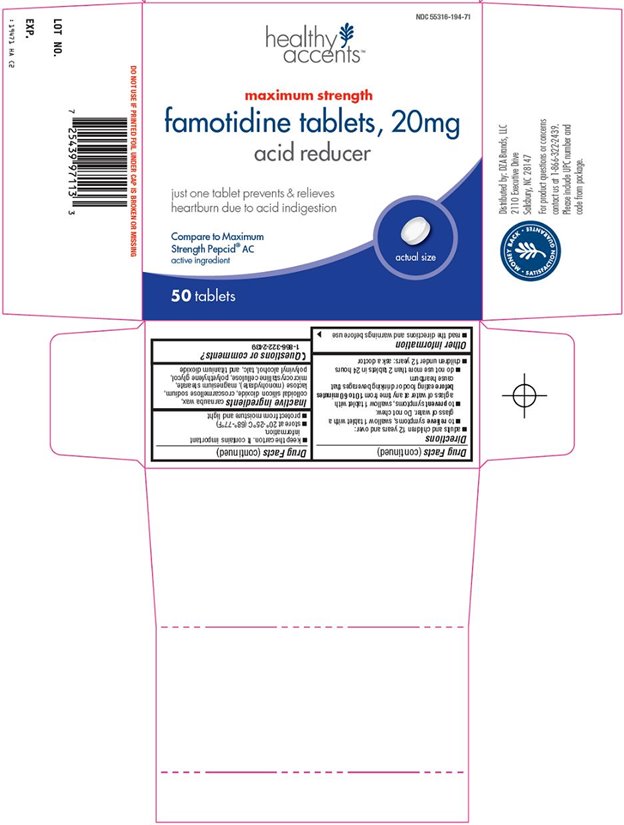
HEALTHY ACCENTS FAMOTIDINE MAXIMUM STRENGTH- famotidine tablet
DZA Brands LLC
----------
DZA Brands, LLC Famotidine Tablets, 20mg Drug Facts
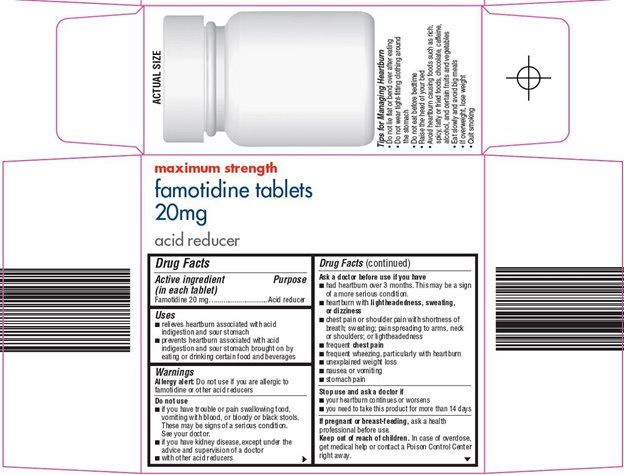
Uses
- relieves heartburn associated with acid indigestion and sour stomach
- prevents heartburn associated with acid indigestion and sour stomach brought on by eating or drinking certain food and beverages
Warnings
Allergy alert: Do not use if you are allergic to famotidine or other acid reducers
Do not use
- if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools. These may be signs of a serious condition. See your doctor.
- if you have kidney disease, except under the advice and supervision of a doctor
- with other acid reducers
Ask a doctor before use if you have
- had heartburn over 3 months. This may be a sign of a more serious condition.
- heartburn with lightheadedness, sweating, or dizziness
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- frequent chest pain
- frequent wheezing, particularly with heartburn
- unexplained weight loss
- nausea or vomiting
- stomach pain
Directions
- adults and children 12 years and over:
- to relieve symptoms, swallow 1 tablet with a glass of water. Do not chew.
- to prevent symptoms, swallow 1 tablet with a glass of water at any time from 10 to 60 minutes before eating food or drinking beverages that cause heartburn
- do not use more than 2 tablets in 24 hours
- children under 12 years: ask a doctor
Other information
- read the directions and warnings before use
- keep the carton. It contains important information.
- store at 20°-25°C (68°-77°F)
- protect from moisture and light
Inactive ingredients
carnauba wax, colloidal silicon dioxide, croscarmellose sodium, lactose (monohydrate), magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, talc, and titanium dioxide
| HEALTHY ACCENTS FAMOTIDINE
MAXIMUM STRENGTH
famotidine tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - DZA Brands LLC (090322194) |
Revised: 11/2019
Document Id: f5b0b8e2-1e75-4cda-9f6e-70d2861590e2
Set id: c3dc2fbc-ac4d-4612-b788-9b6168889f16
Version: 3
Effective Time: 20191112
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.