Vegewax Candleworx Ltd. 44114-520-12 44114-520-50
LASPA by
Drug Labeling and Warnings
LASPA by is a Otc medication manufactured, distributed, or labeled by Vegewax Candleworx Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
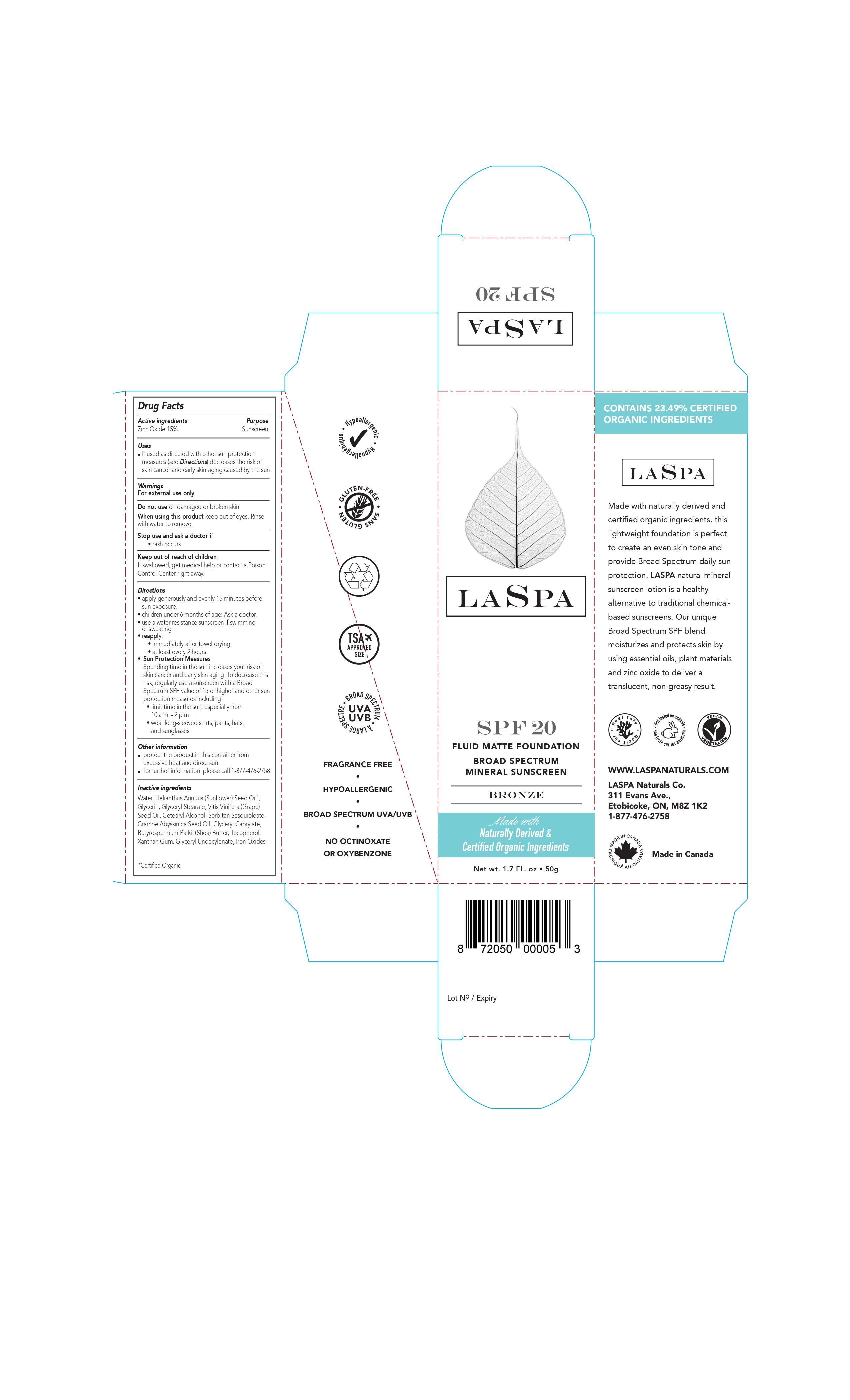
LASPA- spf 20 broad spectrum mineral sunscreen lotion
Vegewax Candleworx Ltd
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Vegewax Candleworx Ltd.
44114-520-12
44114-520-50
If used as directed with other sun protection measures (see Directions) decreases the risk of skin cancer and early skin aging caused by the sun.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
apply generously and evenly 15 minutes before sun exposure.
children under 6 months of age: Ask a doctor.
use a water resistance sunscreen if swimming or sweating
reapply:
immediately after towel drying.
at least every 2 hours
Sun Protection Measures
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
limit time in the sun, especially from 10 a.m. - 2 p.m.
wear long-sleeved shirts, pants, hats, and sunglasses.
Other information
protect the product in this container from excessive heat and direct sun.
for further information please call 1-877-476-2758
Inactive ingredients
Water, Helianthus Annuus (Sunflower) Seed Oil*, Glycerin, Glyceryl Stearate, Vitis Vinifera (Grape) Seed Oil, Cetearyl Alcohol, Sorbitan Sesquioleate, Crambe Abyssinica Seed Oil, Glyceryl Caprylate, Butyrospermum Parkii (Shea) Butter, Tocopherol, Xanthan Gum, Glyceryl Undecylenate, Iron Oxides
*Certified Organic
| LASPA
spf 20 broad spectrum mineral sunscreen lotion |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Vegewax Candleworx Ltd (201050882) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Vegewax Candleworx Ltd | 201050882 | manufacture(44114-520) | |
Trademark Results [LASPA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 LASPA 87567228 not registered Live/Pending |
The Green Cricket Inc. 2017-08-14 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.