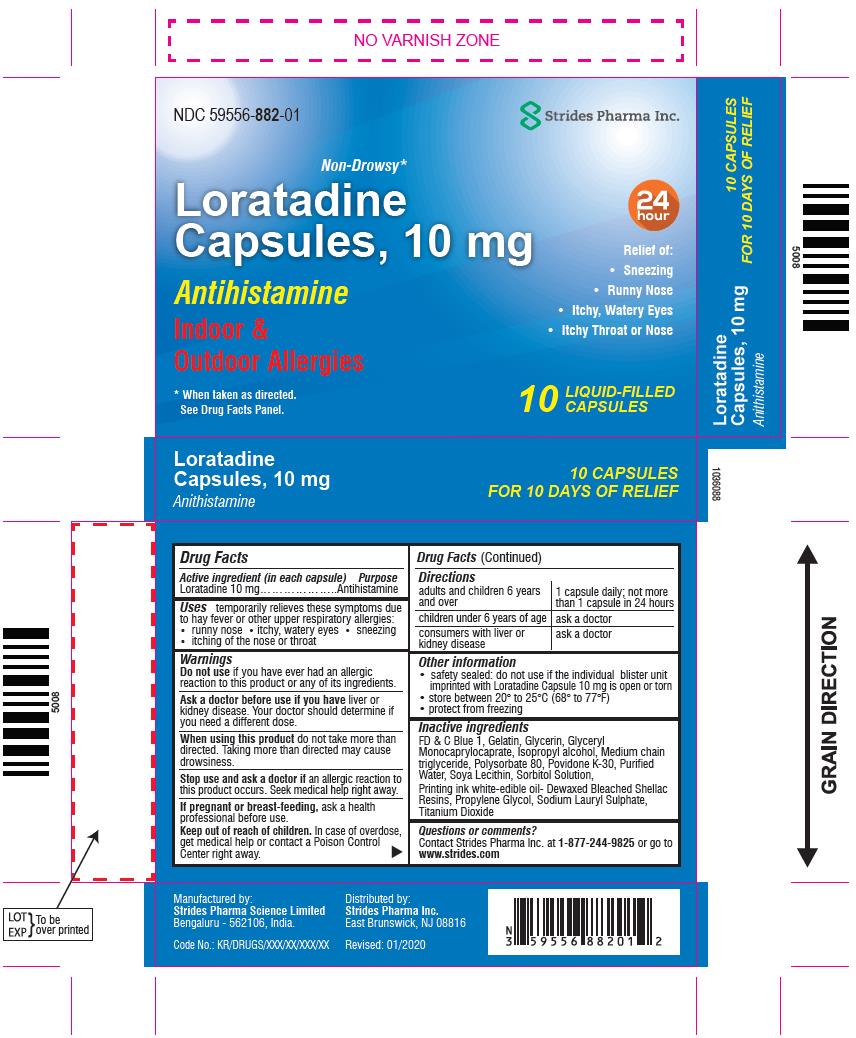
Loratadine Capsules, 10 mg Liquid-Filled Capsules Drug Facts
Loratadine by
Drug Labeling and Warnings
Loratadine by is a Otc medication manufactured, distributed, or labeled by Strides Pharma Inc, Strides Pharma Science Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
LORATADINE - loratadine capsule, liquid filled
Strides Pharma Inc
----------
Loratadine Capsules, 10 mg
Liquid-Filled Capsules
Drug Facts
Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
runny nose
itchy, watery eyes
sneezing
itching of the nose or throat
Warnings
OTC - DO NOT USE
Do not use if you have ever had an allergic reaction to this product or any of its ingredients.
OTC - ASK DOCTOR
Ask a doctor before use if you have liver or kidney disease. Your doctor should determine if you need a different dose.
OTC - WHEN USING
When using this product do not take more than directed. Taking more than directed may cause drowsiness.
OTC - STOP USE
Stop use and ask a doctor if an allergic reaction to this product occurs. Seek medical help right away.
Directions
| adults and children 6 years and over | 1 capsule daily; not more than 1 capsule in 24 hours |
| children under 6 years of age | ask a doctor |
| consumers with liver or kidney disease | ask a doctor |
Other information
- safety sealed: do not use if the individual blister unit imprinted with Loratadine capsule is open or torn
- store between 20° to 25°C (68° to 77°F)
- protect from freezing
Inactive ingredients
FD & C Blue 1, Gelatin, Glycerin, Glyceryl Monocaprylocaprate, Isopropyl alcohol, Medium chain triglyceride, Polysorbate 80, Povidone K-30, Purified Water, Soya Lecithin, Sorbitol Solution,
Printing ink white-edible oil-Dewaxed Bleached Shellac Resins, Propylene Glycol, Sodium Lauryl Sulphate, Titanium Dioxide
.
| LORATADINE
loratadine capsule, liquid filled |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Strides Pharma Inc (078868278) |
| Registrant - Strides Pharma Science Limited (650738743) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Strides Pharma Science Limited | 918513263 | ANALYSIS(59556-882) , MANUFACTURE(59556-882) , PACK(59556-882) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.