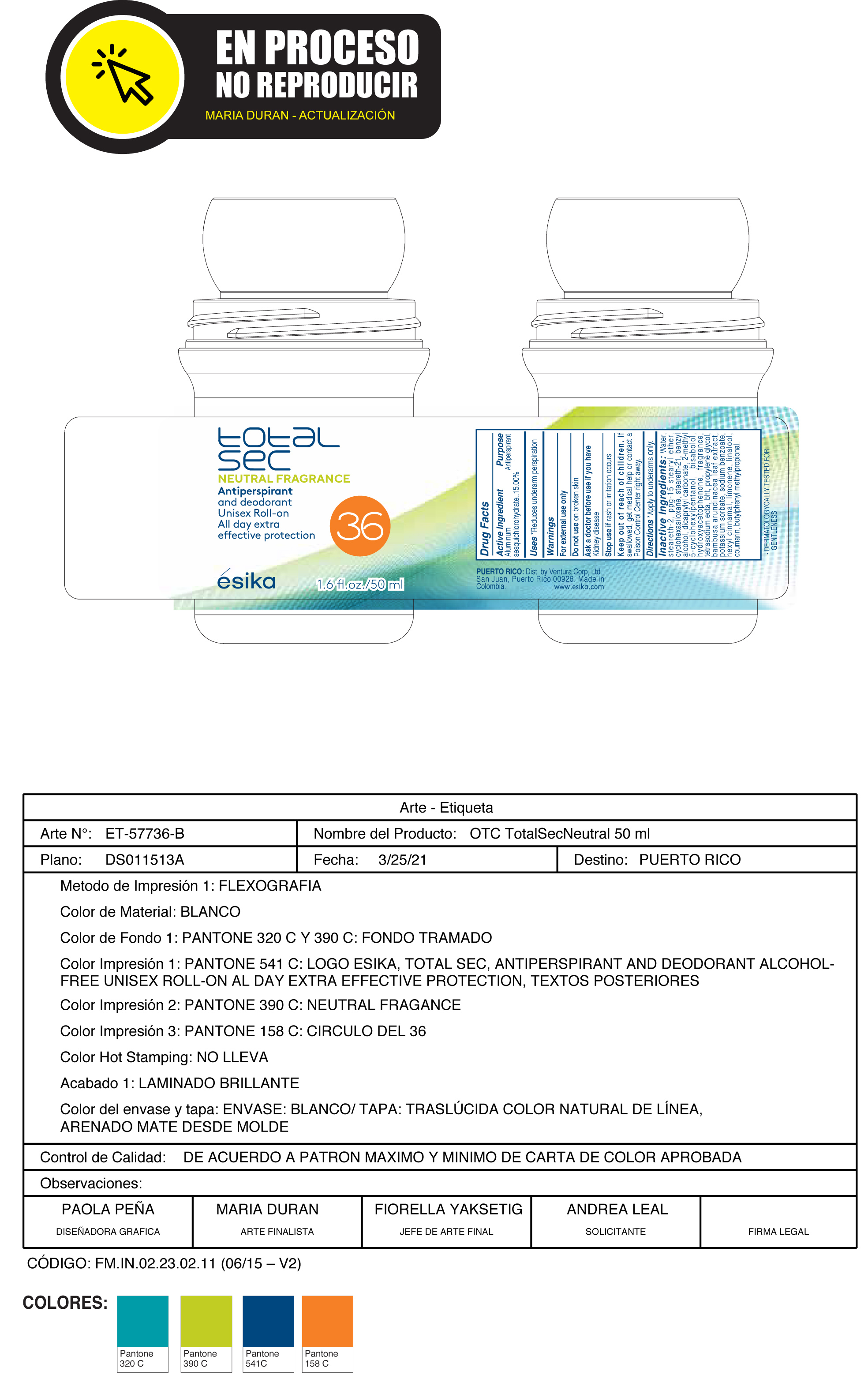
esika total sec NEUTRAL FRAGRANCE Antiperspirant and deodorant Unisex Roll-on All day extra effective protection
esika total sec NEUTRAL FRAGRANCE Antiperspirant and deodorant Unisex Roll-on All day extra effective protection by
Drug Labeling and Warnings
esika total sec NEUTRAL FRAGRANCE Antiperspirant and deodorant Unisex Roll-on All day extra effective protection by is a Otc medication manufactured, distributed, or labeled by Ventura Corporation LTD, BEL STAR S A. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ESIKA TOTAL SEC NEUTRAL FRAGRANCE ANTIPERSPIRANT AND DEODORANT UNISEX ROLL-ON ALL DAY EXTRA EFFECTIVE PROTECTION- aluminum sesquichlorohydrate emulsion
Ventura Corporation LTD
----------
esika total sec NEUTRAL FRAGRANCE Antiperspirant and deodorant Unisex Roll-on All day extra effective protection
Keep out of reach of children.If swallowed, get medical help or contact a Poison Control Center right away.
Warnings
For external use only
Do not useon broken skin
Ask a doctor before use if you havekidney disease
Inactive ingredients:Water, steareth-2, ppg-15 stearyl ether, cyclohexasiloxane, steareth-21, benzyl alcohol, dicaprylyl carbonate, 2-methyl 5-cyclohexylpentanol, bisabolol, hydroxyacetophenone, fragrance, tetrasodium edta, bht, propylene glycol, bambusa arundinacea leaf extract, potassium sorbate, sodium benzoate, hexyl cinnamal, limonene, linalool, coumarin, butylphenyl methylpropional.
| ESIKA TOTAL SEC NEUTRAL FRAGRANCE ANTIPERSPIRANT AND DEODORANT UNISEX ROLL-ON ALL DAY EXTRA EFFECTIVE PROTECTION
aluminum sesquichlorohydrate emulsion |
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Ventura Corporation LTD (602751344) |
| Registrant - BEL STAR S A (880160197) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BEL STAR S A | 880160197 | manufacture(43596-0883) | |