Loratadine by Northwind Pharmaceuticals, LLC LORATADINE tablet
Loratadine by
Drug Labeling and Warnings
Loratadine by is a Prescription medication manufactured, distributed, or labeled by Northwind Pharmaceuticals, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- PURPOSE
- USE(S)
-
WARNINGS
Do not use
If you have ever had an allergic reaction to this product or any of its ingredients.
Ask a doctor before use if you have
Liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
Do not take more than directed. Taking more than directed may cause drowsiness.
Stop use and ask a doctor if
An allergic reaction to this product occurs. Seek medical help right away.
If pregnant or breast-feeding,
Ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
- QUESTIONS
-
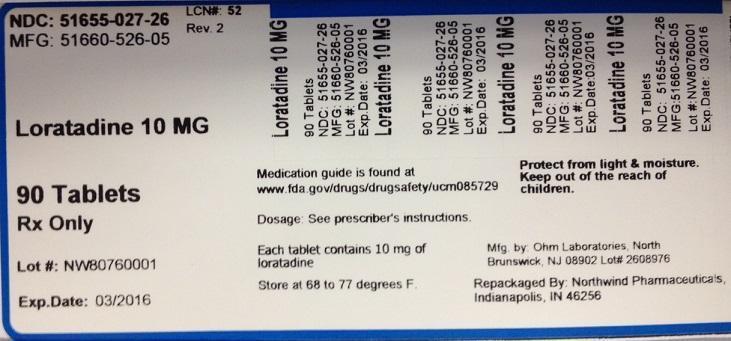
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC: 51655-027-26
MFG: 51660-526-05
Loratadine 10 MG
90 Tablets
Rx Only
Lot#
Exp. Date:
Medication guide is found at www.fda.gov/drugs/drugsafety/ucm085729
Dosage: See prescriber's instructions
Each tablet contains 10 mg of loratadine
Store at 68 to 77 degrees F.
Protect from light and moisture
Keep out of the reach of children.
Mfg. by Ohm Laboratories, North Brunswick, NJ 08902 Lot#
Repackaged by Northwind Pharmaceuticals, Indianapolis, IN 46256
-
INGREDIENTS AND APPEARANCE
LORATADINE
loratadine tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 51655-027(NDC:51660-526) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LORATADINE (UNII: 7AJO3BO7QN) (LORATADINE - UNII:7AJO3BO7QN) LORATADINE 10 mg Product Characteristics Color white Score no score Shape ROUND Size 6mm Flavor Imprint Code RX526 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51655-027-26 90 in 1 BOTTLE, DISPENSING Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076134 10/27/2014 Labeler - Northwind Pharmaceuticals, LLC (036986393) Registrant - Northwind Pharmaceuticals, LLC (036986393) Establishment Name Address ID/FEI Business Operations Northwind Pharmaceuticals, LLC 036986393 repack(51655-027)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.