propylthiouracil by NCS HealthCare of KY, Inc dba Vangard Labs PROPYLTHIOURACIL tablet
propylthiouracil by
Drug Labeling and Warnings
propylthiouracil by is a Prescription medication manufactured, distributed, or labeled by NCS HealthCare of KY, Inc dba Vangard Labs. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
Propylthiouracil (6-propyl-2-thiouracil) is one of the thiocarbamide compounds. It is a white, crystalline substance that has a bitter taste and is very slightly soluble in water.
Each tablet contains propylthiouracil, 50 mg (293.7, µmol), lactose monohydrate, corn starch, colloidal silicon dioxide, povidone, pregelatinized starch, and magnesium stearate.
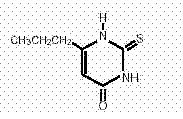
Propylthiouracil is an antithyroid drug administered orally. The molecular weight is 170.23. The molecular formula is C7H10N2OS, and the structural formula is as follows:
-
CLINICAL PHARMACOLOGY
Propylthiouracil inhibits the synthesis of thyroid hormones and thus is effective in the treatment of hyperthyroidism. The drug does not inactivate existing thyroxine and triiodothyronine that are stored in the thyroid or circulating in the blood nor does it interfere with the effectiveness of thyroid hormones given by mouth or by injection.
Propylthiouracil is readily absorbed from the gastrointestinal tract. It is metabolized rapidly and requires frequent administration. Approximately 35% of the drug is excreted in the urine, in intact and in conjugated forms, within 24 hours.
In laboratory animals, various interventions, including propylthiouracil administration, that continuously suppress thyroid function and thereby increase TSH secretion result in thyroid tissue hypertrophy.
-
INDICATIONS AND USAGE
Propylthiouracil is indicated in the medical treatment of hyperthyroidism. Long-term therapy may lead to remission of the disease. Propylthiouracil may also be used to ameliorate hyperthyroidism in preparation for subtotal thyroidectomy or radioactive iodine therapy. Propylthiouracil is also used when thyroidectomy is contraindicated or not advisable.
- CONTRAINDICATIONS
-
WARNINGS
Agranulocytosis is potentially the most serious side effect of propylthiouracil therapy. Patients should be instructed to report any symptoms of agranulocytosis, such as fever or sore throat. Leukopenia, thrombocytopenia, and aplastic anemia (pancytopenia) may also occur. The drug should be discontinued in the presence of agranulocytosis, aplastic anemia (pancytopenia), ANCA-positive vasculitis, hepatitis, interstitial pneumonitis, fever or exfoliative dermatitis. The patient’s bone marrow function should be monitored.
Propylthiouracil can cause fetal harm when administered to a pregnant woman. Because the drug readily crosses placental membranes and can induce goiter and even cretinism in the developing fetus, it is important that a sufficient, but not excessive, dose be given. In many pregnant women, the thyroid dysfunction diminishes as the pregnancy proceeds; consequently, a reduction of dosage may be possible. In some instances, propylthiouracil can be withdrawn 2 or 3 weeks before delivery.
If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be warned of the potential hazard to the fetus.
Postpartum patients receiving propylthiouracil should not nurse their babies.
Rare reports exist of severe hepatic reactions including encephalopathy, fulminant hepatic necrosis and death in patients receiving propylthiouracil. Symptoms suggestive of hepatic dysfunction (anorexia, pruritus, right upper quadrant pain, etc.) should prompt evaluation of liver function. Treatment with propylthiouracil should be discontinued promptly in the event of clinically significant evidence of liver abnormality, including hepatic transaminases in excess of 3 times the upper limit of normal.
-
PRECAUTIONS
General
Patients who receive propylthiouracil should be under close surveillance and should be impressed with the necessity of reporting immediately any evidence of illness, particularly sore throat, skin eruptions, fever, headache, or general malaise. In such cases, white-blood-cell and differential counts should be made to determine whether agranulocytosis has developed. Particular care should be exercised with patients who are receiving additional drugs known to cause agranulocytosis.
Information for Patients
Patients should be advised that if they become pregnant during therapy or intend to become pregnant, they should contact their physician immediately about the desirability of discontinuing the drug. They also should not use propylthiouracil while nursing.
Patients should report immediately any evidence of illness, particularly sore throat, skin eruptions, fever, headache, or general malaise. They also should report symptoms suggestive of hepatic dysfunction (anorexia, pruritus, right upper quadrant pain, etc).
Laboratory Tests
Because propylthiouracil may cause hypoprothrombinemia and bleeding, prothrombin time should be monitored during therapy with the drug, especially before surgical procedures. Thyroid function tests should be monitored periodically during therapy. Once clinical evidence of hyperthyroidism has resolved, the finding of an elevated serum TSH indicates that a lower maintenance dose of propylthiouracil should be employed.
Drug Interactions
Anticoagulants (Oral): The activity of anticoagulants may be potentiated by anti-vitamin-K activity attributed to propylthiouracil.
β-Adrenergic Blocking Agents: Hyperthyroidism may cause an increased clearance of beta blockers with a high extraction ratio. A dose reduction of beta-adrenergic blockers may be needed when a hyperthyroid patient becomes euthyroid.
Digitalis Glycosides: Serum digitalis levels may be increased when hyperthyroid patients on a stable digitalis glycoside regimen become euthyroid; a reduced dosage of digitalis glycosides may be required.
Theophylline: Theophylline clearance may decrease when hyperthyroid patients on a stable theophylline regimen become euthyroid; a reduced dose of theophylline may be needed.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Laboratory animals treated with propylthiouracil for > 1 year have demonstrated thyroid hyperplasia and carcinoma formation.1 Such animal findings are seen with continuous suppression of thyroid function by sufficient doses of a variety of antithyroid agents, as well as in dietary iodine deficiency, subtotal thyroidectomy, and implantation of autonomous thyrotropic hormone-secreting pituitary tumors. Pituitary adenomas have also been described.
Nursing Mothers
The drug appears in human milk and is contraindicated in nursing mothers. (See CONTRAINDICATIONSand WARNINGS.)
Pediatric Use
Safety and effectiveness in pediatric patients below the age of 6 have not been established. For pediatric patients 6 years of age and older, see DOSAGE AND ADMINISTRATION.
-
ADVERSE REACTIONS
Major adverse reactions (much less common than the minor adverse reactions) include inhibition of myelopoiesis (agranulocytosis, granulopenia, and thrombocytopenia), aplastic anemia, drug fever, a lupus-like syndrome (including splenomegaly and vasculitis), hepatitis, periarteritis and hypoprothrombinemia and bleeding. Nephritis, glomerulonephritis, interstitial pneumonitis, exfoliative dermatitis, and erythema nodosum have been reported. Reports of a vasculitic syndrome associated with the presence of anti-neutrophilic cytoplasmic antibiodies (ANCA) have also been received. Manifestations of ANCA-positive vasculitis may include rapidly progressive glomerulonephritis (crescentic and pauci-immune necrotizing glomerulonephritis) sometimes leading to acute renal failure; fever; pulmonary infiltrates or alveolar hemorrhage; skin ulcers; and leucocytoclastic vasculitis.
Minor adverse reactions include skin rash, urticaria, nausea, vomiting, epigastric distress, arthralgia, paresthesia, loss of taste, taste perversion, abnormal loss of hair, myalgia, headache, pruritus, drowsiness, neuritis, edema, vertigo, skin pigmentation, jaundice, sialadenopathy, and lymphadenopathy.
It should be noted that about 10% of patients with untreated hyperthyroidism have leukopenia (white-blood cell count of less than 4,000/mm3), often with relative granulopenia.
-
OVERDOSAGE
Signs And Symptoms:Nausea, vomiting, epigastric distress, headache, fever, arthralgia, pruritus, edema, and pancytopenia. Agranulocytosis is the most serious effect. Rarely, exfoliative dermatitis, hepatitis, neuropathies, or CNS stimulation or depression may occur.
No information is available on the following: LD50; concentration of propylthiouracil in biologic fluids associated with toxicity and/or death; the amount of drug in a single dose usually associated with symptoms of overdosage; or the amount of propylthiouracil in a single dose likely to be life-threatening.
Treatment:To obtain up-to-date information about the treatment of overdose, a good resource is your certified Regional Poison Control Center. Telephone numbers of certified poison control centers are listed in the Physicians’ Desk Reference (PDR). In managing overdosage, consider the possibility of multiple drug overdoses, interaction among drugs, and unusual drug kinetics in your patient.
Protect the patient’s airway and support ventilation and perfusion. Meticulously monitor and maintain, within acceptable limits, the patient’s vital signs, blood gases, serum electrolytes, etc. The patient’s bone marrow function should be monitored. Absorption of drugs from the gastrointestinal tract may be decreased by giving activated charcoal, which, in many cases, is more effective than emesis or lavage; consider charcoal instead of or in addition to gastric emptying. Repeated doses of charcoal over time may hasten elimination of some drugs that have been absorbed. Safeguard the patient’s airway when employing gastric emptying or charcoal.
Forced diuresis, peritoneal dialysis, hemodialysis, or charcoal hemoperfusion have not been established as beneficial for an overdose of propylthiouracil.
-
DOSAGE AND ADMINISTRATION
Propylthiouracil is administered orally. The total daily dosage is usually given in 3 equal doses at approximately 8-hour intervals.
Adults:The initial dosage is 300 mg daily. In patients with severe hyperthyroidism, very large goiters, or both, the beginning dosage usually should be 400 mg daily; an occasional patient will require 600 to 900 mg/daily initially. The usual maintenance dosage is 100 to 150 mg daily.
Pediatric Patients: For pediatric patients 6 to 10 years of age, the initial dosage is 50 to 150 mg daily. For pediatric patients 10 years and over, the initial dosage is 150 to 300 mg daily. The maintenance dosage is determined by the response of the patient.
-
HOW SUPPLIED
50 mg — Each white, round, tablet imprinted with
 on one side and 348 and partial bisect on the other side contains 50 mg of Propylthiouracil, USP. Tablets are supplied in blisterpacks of 30 (NDC: 0615-7583-39).
on one side and 348 and partial bisect on the other side contains 50 mg of Propylthiouracil, USP. Tablets are supplied in blisterpacks of 30 (NDC: 0615-7583-39). -
REFERENCES
1. International Agency for Research on Cancer. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. 1974; 7:67-76.
Dispense in a well-closed container as defined in the USP.
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F).
Manufactured by:
Actavis Elizabeth LLC
200 Elmora Avenue, Elizabeth, NJ 07207 USA
40-8756
Revised — January 2008
- PACKAGE LABEL PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PROPYLTHIOURACIL
propylthiouracil tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0615-7583(NDC:0228-2348) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PROPYLTHIOURACIL (UNII: 721M9407IY) (PROPYLTHIOURACIL - UNII:721M9407IY) PROPYLTHIOURACIL 50 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) STARCH, CORN (UNII: O8232NY3SJ) COLLOIDAL SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POVIDONE (UNII: FZ989GH94E) STARCH, CORN (UNII: O8232NY3SJ) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color WHITE Score no score Shape ROUND Size 8mm Flavor Imprint Code 348 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0615-7583-39 30 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA080172 01/09/1973 Labeler - NCS HealthCare of KY, Inc dba Vangard Labs (050052943) Establishment Name Address ID/FEI Business Operations NCS HealthCare of KY, Inc dba Vangard Labs 050052943 RELABEL, REPACK
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.