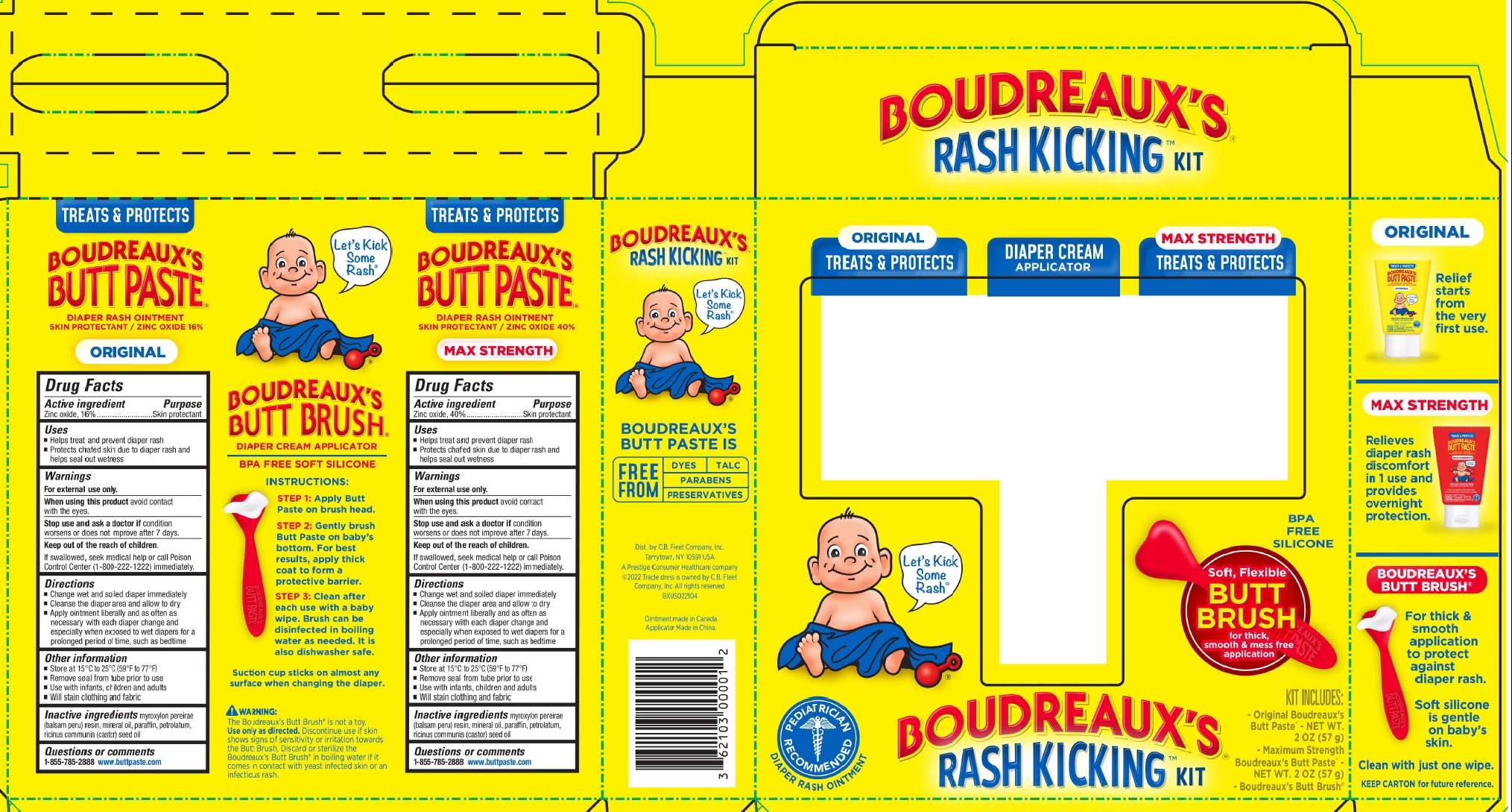
Active ingredient
Zinc oxide, 40%
Uses
- Helps treat and prevent diaper rash
- Protects chafed skin due to diaper rash and helps seal out wetness
Warnings
When using this product
- avoid contact with the eyes
Stop use and ask a doctor if
- condition worsens or does not improve after 7 days
Keep out of reach of children
- to prevent accidental ingestion
- If swallowed, seek medical help or call Poison Control Center (1-800-222-1222) immediately
Directions
- Change wet and soiled diaper immediately
- Cleanse the diaper area and allow to dry
- Apply ointment liberally and as often as neessary with each diaper change and especially when exposed to wet diapers for a prolonged period of time, such as bedtime
Other information
- Store at 15°C-25°C (59°F-77°F)
- Remove foil seal from tube's tip before use
- Use with infants, children and adults
- Will stain clothing and fabric
Inactive ingredients
myroxylon pereirae (balsam peru) resin, mineral oil, paraffin, petrolatum, ricinus communis (castor) seed oil
Questions?
1-855-785-2888
www.buttpaste.com
Active ingredient
Zinc oxide, 16%
Uses
- Helps treat and prevent diaper rash
- Protects chafed skin due to diaper rash and helps seal out wetness
Warnings
When using this product
- avoid contact with the eyes
Stop use and ask a doctor if
- condition worsens or does not improve after 7 days
Keep out of reach of children
- to prevent accidental ingestion
- If swallowed, seek medical help or call Poison Control Center (1-800-222-1222) immediately
Directions
- Change wet and soiled diaper immediately
- Cleanse the diaper area and allow to dry
- Apply ointment liberally and as often as neessary with each diaper change and especially when exposed to wet diapers for a prolonged period of time, such as bedtime
Other information
- Store at 15°C-25°C (59°F-77°F)
- Remove foil seal from tube's tip before use
- Use with infants, children and adults
- Will stain clothing and fabric
Inactive ingredients
myroxylon pereirae (balsam peru) resin, mineral oil, paraffin, petrolatum, ricinus communis (castor) seed oil
Questions?
1-855-785-2888
www.buttpaste.com
Package Labeling:63471-3000-0
Package Labeling:63471-3001-2

Package Labeling:63471-3002-2



