Antibacterial Wet Wipes by Positive Promotions Inc. Antibacterial Wet Wipes
Antibacterial Wet Wipes by
Drug Labeling and Warnings
Antibacterial Wet Wipes by is a Otc medication manufactured, distributed, or labeled by Positive Promotions Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
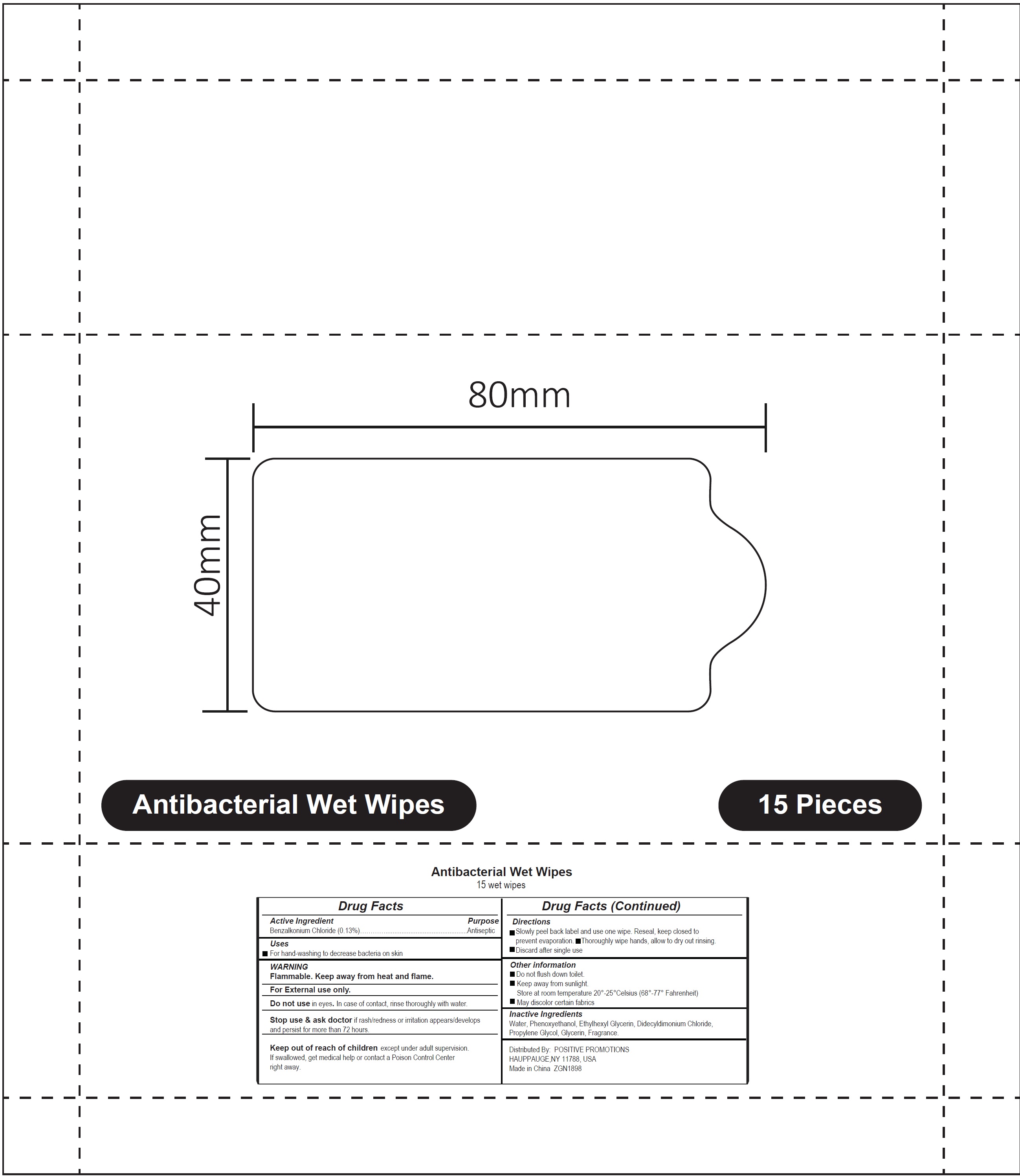
ANTIBACTERIAL WET WIPES- benzalkonium chloride cloth
Positive Promotions Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Antibacterial Wet Wipes
WARNING
Flammable. Keep away from heat and flame.
For External use only.
Directions
- Slowly peel back label and use one wipe. Reseal, keep closed to prevent evaporation.
- Thoroughly wipe hands, allow to dry out rinsing.
- Discard after single use
Other information
- Do not flush down toilet.
- Keep away from sunlight. Store at room temperature 20°-25°Celsius (68°-77° Fahrenheit)
- May discolor certain fabrics
| ANTIBACTERIAL WET WIPES
benzalkonium chloride cloth |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Positive Promotions Inc. (002401719) |
Revised: 2/2022
Document Id: d79c20a4-688c-0394-e053-2a95a90a760b
Set id: c46d9c7f-4452-0aed-e053-2995a90a503e
Version: 2
Effective Time: 20220209