TICAGRELOR tablet, film coated
Ticagrelor by
Drug Labeling and Warnings
Ticagrelor by is a Prescription medication manufactured, distributed, or labeled by Hisun Pharmaceuticals USA, Inc., Hisun Pharmaceutical (Hangzhou) Co., Ltd., Hanhui Pharmaceuticals Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TICAGRELOR TABLETS safely and effectively. See full prescribing information for TICAGRELOR TABLETS.
TICAGRELOR tablets, for oral use
Initial U.S. Approval: 2011See full prescribing information for complete boxed warning.
- Ticagrelor, like other antiplatelet agents, can cause significant, sometimes fatal bleeding. ( 5.1, 6.1)
- Do not use ticagrelor in patients with active pathological bleeding or a history of intracranial hemorrhage. ( 4.1, 4.2)
- Do not start ticagrelor in patients undergoing urgent coronary artery bypass graft surgery (CABG). ( 5.1, 6.1)
If possible, manage bleeding without discontinuing ticagrelor. Stopping ticagrelor increases the risk of subsequent cardiovascular events. ( 5.2)
INDICATIONS AND USAGE
Ticagrelor is a P2Y 12 platelet inhibitor indicated
- to reduce the risk of a first MI or stroke in patients with coronary artery disease (CAD) at high risk for such events. While use is not limited to this setting, the efficacy of Ticagrelor was established in a population with type 2 diabetes mellitus (T2DM). ( 1.2)
- to reduce the risk of stroke in patients with acute ischemic stroke (NIH Stroke Scale score ≤5) or high-risk transient ischemic attack (TIA). ( 1.3)
DOSAGE AND ADMINISTRATION
- Patients with CAD and No Prior Stroke or MI
Administer 60 mg ticagrelor tablets twice daily. ( 2.3)
- Acute Ischemic Stroke
Initiate treatment with 180 mg loading dose of ticagrelor tablets then continue with 90 mg twice daily for up to 30 days. ( 2.4)
Use ticagrelor tablets with a daily maintenance dose of aspirin of 75-100 mg. ( 2)
DOSAGE FORMS AND STRENGTHS
- 90 mg tablets ( 3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Dyspnea was reported more frequently with ticagrelor than with control agents in clinical trials. Dyspnea from ticagrelor is self-limiting. ( 5.3)
- Severe Hepatic Impairment: Likely increase in exposure to ticagrelor. ( 5.5)
- Laboratory Test Interference: False negative platelet functional test results have been reported for Heparin Induced Thrombocytopenia (HIT). Ticagrelor is not expected to impact PF4 antibody testing for HIT ( 5.7).
ADVERSE REACTIONS
Most common adverse reactions (>5%) are bleeding and dyspnea. ( 5.1, 5.3, 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Hisun Pharmaceuticals USA, Inc. at 1-855-554-4786 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Avoid use with strong CYP3A inhibitors or CYP3A inducers. ( 7.1, 7.2)
- Opioids: Decreased exposure to ticagrelor. Consider use of parenteral anti-platelet agent. ( 7.3)
- Patients receiving more than 40 mg per day of simvastatin or lovastatin may be at increased risk of statin-related adverse effects. ( 7.4)
- Rosuvastatin plasma concentrations may increase. Monitor for statin-related adverse effects. ( 7.4)
- Monitor digoxin levels with initiation of or any change in ticagrelor. ( 7.5)
USE IN SPECIFIC POPULATIONS
Lactation: Breastfeeding not recommended ( 8.2)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 10/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: BLEEDING RISK
1 INDICATIONS AND USAGE
1.2 Coronary Artery Disease but No Prior Stroke or Myocardial Infarction
1.3 Acute Ischemic Stroke or Transient Ischemic Attack (TIA)
2 DOSAGE AND ADMINISTRATION
2.1 General Instructions
2.3 Coronary Artery Disease but No Prior Stroke or Myocardial Infarction
2.4 Acute Ischemic Stroke or Transient Ischemic Attack (TIA)
2.4 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 History of Intracranial Hemorrhage
4.2 Active Bleeding
4.3 Hypersensitivity
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Bleeding
5.2 Discontinuation of Ticagrelor in Patients Treated for Coronary Artery Disease
5.3 Dyspnea
5.4 Bradyarrhythmias
5.5 Severe Hepatic Impairment
5.6 Central Sleep Apnea
5.7 Laboratory Test Interferences
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Strong CYP3A Inhibitors
7.2 Strong CYP3A Inducers
7.3 Opioids
7.4 Simvastatin, Lovastatin, Rosuvastatin
7.5 Digoxin
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.2 Coronary Artery Disease but No Prior Stroke or Myocardial Infarction
14.3 Acute Ischemic Stroke or Transient Ischemic Attack (TIA)
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: BLEEDING RISK
- Ticagrelor, like other antiplatelet agents, can cause significant, sometimes fatal bleeding ( 5.1, 6.1>).
- Do not use ticagrelor in patients with active pathological bleeding or a history of intracranial hemorrhage ( 4.1, 4.2).
- Do not start ticagrelor in patients undergoing urgent coronary artery bypass graft surgery (CABG) ( 5.1, 6.1).
If possible, manage bleeding without discontinuing ticagrelor. Stopping ticagrelor increases the risk of subsequent cardiovascular events ( 5.2).
-
1 INDICATIONS AND USAGE
1.2 Coronary Artery Disease but No Prior Stroke or Myocardial Infarction
Ticagrelor is indicated to reduce the risk of a first MI or stroke in patients with coronary artery disease (CAD) at high risk for such events [see Clinical Studies ( 14.2)] . While use is not limited to this setting, the efficacy of Ticagrelor was established in a population with type 2 diabetes mellitus (T2DM).
1.3 Acute Ischemic Stroke or Transient Ischemic Attack (TIA)
Ticagrelor is indicated to reduce the risk of stroke in patients with acute ischemic stroke (NIH Stroke Scale score ≤5) or high-risk transient ischemic attack (TIA) [see Clinical Studies ( 14.3)] .
-
2 DOSAGE AND ADMINISTRATION
2.1 General Instructions
Advise patients who miss a dose of ticagrelor tablets to take their next dose at its scheduled time.
For patients who are unable to swallow tablets whole, ticagrelor tablets can be crushed, mixed with water, and drunk. The mixture can also be administered via a nasogastric tube (CH8 or greater) [see Clinical Pharmacology ( 12.3)].
Do not administer ticagrelor tablets with another oral P2Y 12 platelet inhibitor.
Avoid aspirin at doses higher than recommended [see Clinical Studies ( 14.1)].
2.3 Coronary Artery Disease but No Prior Stroke or Myocardial Infarction
Administer 60 mg of ticagrelor tablets twice daily.
Generally, use ticagrelor tablets with a daily maintenance dose of aspirin of 75 mg to 100 mg [see Clinical Studies ( 14)] .
2.4 Acute Ischemic Stroke or Transient Ischemic Attack (TIA)
Initiate treatment with a 180 mg loading dose of ticagrelor tablets and then continue with 90 mg twice daily for up to 30 days.
Administer the first maintenance dose 6 to 12 hours after the loading dose.
Use ticagrelor tablets with a loading dose of aspirin (300 mg to 325 mg) and a daily maintenance dose of aspirin of 75 mg to 100 mg [see Clinical Studies ( 14)] .
2.4 Administration
A patient who misses a dose of ticagrelor tablets should take one tablet (their next dose) at its scheduled time.
For patients who are unable to swallow tablets whole, ticagrelor tablets can be crushed, mixed with water and drunk. The mixture can also be administered via a nasogastric tube (CH8 or greater) [see Clinical Pharmacology ( 12.3)].
Do not administer ticagrelor tablets with another oral P2Y 12 platelet inhibitor. - 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
4.1 History of Intracranial Hemorrhage
Ticagrelor is contraindicated in patients with a history of intracranial hemorrhage (ICH) because of a high risk of recurrent ICH in this population [see Clinical Studies ( 14.1, 14.2)] .
-
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Bleeding
Drugs that inhibit platelet function including ticagrelor increase the risk of bleeding [see Warnings and Precautions ( 5.2) and Adverse Reactions ( 6.1)] .
Patients treated for acute ischemic stroke or TIA
Patients at NIHSS >5 and patients receiving thrombolysis were excluded from THALES and use of ticagrelor in such patients is not recommended.
5.2 Discontinuation of Ticagrelor in Patients Treated for Coronary Artery Disease
Discontinuation of ticagrelor will increase the risk of myocardial infarction, stroke, and death in patients being treated for coronary artery disease. If ticagrelor must be temporarily discontinued (e.g., to treat bleeding or for significant surgery), restart it as soon as possible. When possible, interrupt therapy with ticagrelor for five days prior to surgery that has a major risk of bleeding. Resume ticagrelor as soon as hemostasis is achieved.
5.3 Dyspnea
In clinical trials, about 21% (THEMIS) of patients treated with ticagrelor developed dyspnea. Dyspnea was usually mild to moderate in intensity and often resolved during continued treatment but led to study drug discontinuation in 1.0% (THALES) and 6.9% (THEMIS) of patients.
If a patient develops new, prolonged, or worsened dyspnea that is determined to be related to ticagrelor, no specific treatment is required; continue ticagrelor without interruption if possible. In the case of intolerable dyspnea requiring discontinuation of ticagrelor, consider prescribing another antiplatelet agent.
5.4 Bradyarrhythmias
Ticagrelor can cause ventricular pauses [see Adverse Reactions ( 6.1)] . Bradyarrhythmias including AV block have been reported in the postmarketing setting. Patients with a history of sick sinus syndrome, 2 nd or 3 rd degree AV block or bradycardia-related syncope not protected by a pacemaker were excluded from clinical studies and may be at increased risk of developing bradyarrhythmias with ticagrelor.
5.5 Severe Hepatic Impairment
Avoid use of ticagrelor in patients with severe hepatic impairment. Severe hepatic impairment is likely to increase serum concentration of ticagrelor. There are no studies of ticagrelor patients with severe hepatic impairment [see Clinical Pharmacology ( 12.3)] .
5.6 Central Sleep Apnea
Central sleep apnea (CSA) including Cheyne-Stokes respiration (CSR) has been reported in the post-marketing setting in patients taking ticagrelor, including recurrence or worsening of CSA/CSR following rechallenge. If central sleep apnea is suspected, consider further clinical assessment.
5.7 Laboratory Test Interferences
False negative functional tests for Heparin Induced Thrombocytopenia (HIT)
Ticagrelor has been reported to cause false negative results in platelet functional tests (including the heparin-induced platelet aggregation (HIPA) assay) for patients with Heparin Induced Thrombocytopenia (HIT). This is related to inhibition of the P2Y 12-receptor on the healthy donor platelets in the test by ticagrelor in the affected patient’s serum/plasma. Information on concomitant treatment with ticagrelor is required for interpretation of HIT functional tests. Based on the mechanism of ticagrelor interference, ticagrelor is not expected to impact PF4 antibody testing for HIT.
-
6 ADVERSE REACTIONS
The following adverse reactions are also discussed elsewhere in the labeling:
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Ticagrelor has been evaluated for safety in more than 58,000 patients.
Bleeding in THEMIS (Prevention of major CV events in patients with CAD and Type 2 Diabetes Mellitus)
The Kaplan-Meier curve of time to first TIMI Major bleeding event is presented in Figure 3.
Figure 3 - Time to first TIMI Major bleeding event (THEMIS)
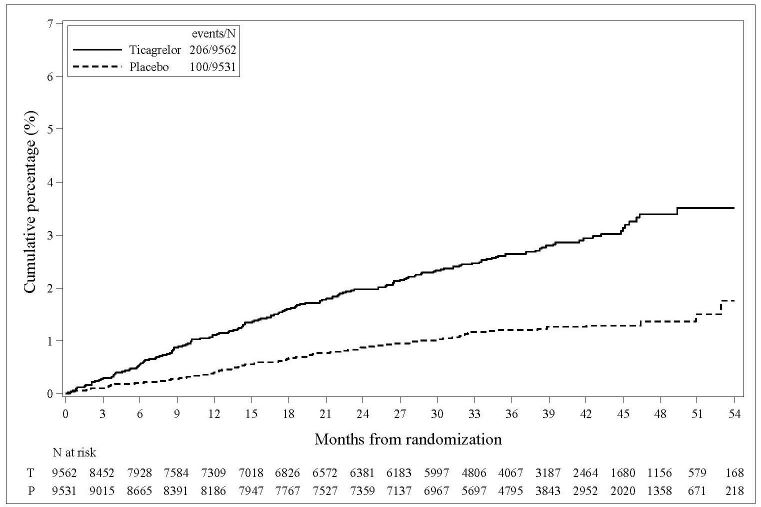
T = Ticagrelor; P = Placebo; N = Number of patients
The bleeding events in THEMIS are shown below in Table 6.
Table 6 – Bleeding events (THEMIS)
Ticagrelor
N=9562
Placebo
N=9531
Events / 1000 patient years
Events / 1000 patient years
TIMI Major
9
4
TIMI Major or Minor
12
5
TIMI Major or Minor or Requiring medical attention
46
18
Fatal bleeding
1
0
Intracranial hemorrhage
3
2
Bleeding in THALES (Reduction in risk of stroke in patients with acute ischemic stroke or TIA)
The Kaplan-Meier curve of time course of GUSTO severe bleeding events is presented in Figure 4.Figure 4 - Time course of GUSTO severe bleeding events
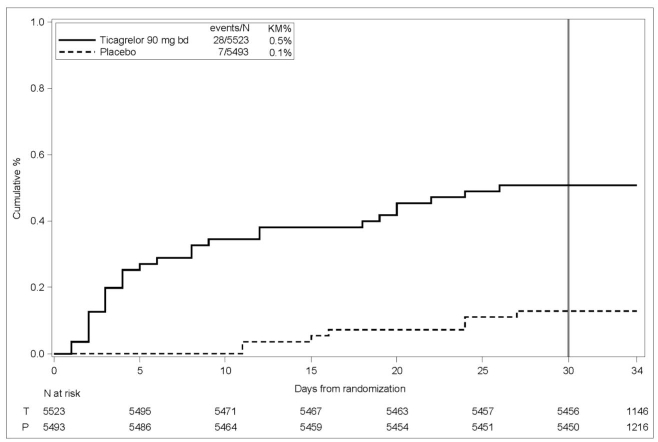
KM%: Kaplan-Meier percentage evaluated at Day 30; T = Ticagrelor; P = placebo; N = Number of patients
GUSTO Severe: Any one of the following: fatal bleeding, intracranial bleeding (excluding asymptomatic hemorrhagic transformations of ischemic brain infarctions and excluding microhemorrhages < 10 mm evident only on gradient-echo magnetic resonance imaging), bleeding that caused hemodynamic compromise requiring intervention (eg, systolic blood pressure <90 mmg Hg that required blood or fluid replacement, or vasopressor/inotropic support, or surgical intervention).Intracranial bleeding and fatal bleeding in THALES: In total, there were 21 intracranial hemorrhages (ICHs) for ticagrelor and 6 ICHs for placebo. Fatal bleedings, almost all ICH, occurred in 11 for ticagrelor and in 2 for placebo.
Bradycardia
THEMIS and THALES excluded patients at increased risk of bradycardic events (e.g., patients who have sick sinus syndrome, 2 nd or 3 rd degree AV block, or bradycardic-related syncope and not protected with a pacemaker).
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of ticagrelor. Because these reactions are reported voluntarily from a population of an unknown size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and lymphatic system disorders: Thrombotic Thrombocytopenic Purpura (TTP) has been rarely reported with the use of ticagrelor. TTP is a serious condition which can occur after a brief exposure (<2 weeks) and requires prompt treatment.
Immune system disorders: Hypersensitivity reactions including angioedema [see Contraindications ( 4.3)].
Respiratory Disorders: Central sleep apnea, Cheyne-Stokes respiration
Skin and subcutaneous tissue disorders: Rash
-
7 DRUG INTERACTIONS
7.1 Strong CYP3A Inhibitors
Strong CYP3A inhibitors substantially increase ticagrelor exposure and so increase the risk of dyspnea, bleeding, and other adverse events. Avoid use of strong inhibitors of CYP3A (e.g., ketoconazole, itraconazole, voriconazole, clarithromycin, nefazodone, ritonavir, saquinavir, nelfinavir, indinavir, atazanavir and telithromycin) [see Clinical Pharmacology (12.3)].
7.2 Strong CYP3A Inducers
Strong CYP3A inducers substantially reduce ticagrelor exposure and so decrease the efficacy of ticagrelor. Avoid use with strong inducers of CYP3A (e.g., rifampin, phenytoin, carbamazepine and phenobarbital) [see Clinical Pharmacology (12.3)].
7.3 Opioids
As with other oral P2Y 12 inhibitors, co-administration of opioid agonists delay and reduce the absorption of ticagrelor and its active metabolite presumably because of slowed gastric emptying [see Clinical Pharmacology (12.3)]. . Consider the use of a parenteral anti-platelet agent in acute coronary syndrome patients requiring co-administration of morphine or other opioid agonists.
7.4 Simvastatin, Lovastatin, Rosuvastatin
Ticagrelor increases serum concentrations of simvastatin and lovastatin because these drugs are metabolized by CYP3A4. Avoid simvastatin and lovastatin doses greater than 40 mg [see Clinical Pharmacology ( 12.3)] .
Ticagrelor increases serum concentration of rosuvastatin because rosuvastatin is a BCRP substrate [see Clinical Pharmacology ( 12.3)] .
7.5 Digoxin
Ticagrelor inhibits the P-glycoprotein transporter; monitor digoxin levels with initiation of or change in ticagrelor therapy [see Clinical Pharmacology ( 12.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from case reports with ticagrelor use in pregnant women have not identified a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. Ticagrelor given to pregnant rats and pregnant rabbits during organogenesis caused structural abnormalities in the offspring at maternal doses about 5 to 7 times the maximum recommended human dose (MRHD) based on body surface area. When ticagrelor was given to rats during late gestation and lactation, pup death and effects on pup growth were seen at approximately 10 times the MRHD (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
In reproductive toxicology studies, pregnant rats received ticagrelor during organogenesis at doses from 20 to 300 mg/kg/day. 20 mg/kg/day is approximately the same as the MRHD of 90 mg twice daily for a 60 kg human on a mg/m 2 basis. Adverse outcomes in offspring occurred at doses of 300 mg/kg/day (16.5 times the MRHD on a mg/m 2 basis) and included supernumerary liver lobe and ribs, incomplete ossification of sternebrae, displaced articulation of pelvis, and misshapen/misaligned sternebrae. At the mid-dose of 100 mg/kg/day (5.5 times the MRHD on a mg/m 2 basis), delayed development of liver and skeleton was seen. When pregnant rabbits received ticagrelor during organogenesis at doses from 21 to 63 mg/kg/day, fetuses exposed to the highest maternal dose of 63 mg/kg/day (6.8 times the MRHD on a mg/m 2 basis) had delayed gall bladder development and incomplete ossification of the hyoid, pubis and sternebrae occurred.
In a prenatal/postnatal study, pregnant rats received ticagrelor at doses of 10 to 180 mg/kg/day during late gestation and lactation. Pup death and effects on pup growth were observed at 180 mg/kg/day (approximately 10 times the MRHD on a mg/m 2 basis). Relatively minor effects such as delays in pinna unfolding and eye opening occurred at doses of 10 and 60 mg/kg (approximately one-half and 3.2 times the MRHD on a mg/m 2 basis).
8.2 Lactation
Risk Summary
There are no data on the presence of ticagrelor or its metabolites in human milk, the effects on the breastfed infant, or the effects on milk production. Ticagrelor and its metabolites were present in rat milk at higher concentrations than in maternal plasma. When a drug is present in animal milk, it is likely that the drug will be present in human milk. Breastfeeding is not recommended during treatment with ticagrelor.
8.4 Pediatric Use
The safety and effectiveness of ticagrelor have not been established in pediatric patients.
Pediatric use information describing a clinical study in which efficacy was not demonstrated is approved for AstraZeneca Pharmaceuticals LP’s BRILINTA® (ticagrelor) tablets. However, due to AstraZeneca Pharmaceuticals LP’s marketing exclusivity rights, this drug product is not labeled with that information.
8.5 Geriatric Use
About half of the patients in THEMIS and THALES were ≥65 years of age and at least 15% were ≥75 years of age. No overall differences in safety or effectiveness were observed between elderly and younger patients.
8.6 Hepatic Impairment
Ticagrelor is metabolized by the liver and impaired hepatic function can increase risks for bleeding and other adverse events. Avoid use of ticagrelor in patients with severe hepatic impairment. There is limited experience with ticagrelor in patients with moderate hepatic impairment; consider the risks and benefits of treatment, noting the probable increase in exposure to ticagrelor. No dosage adjustment is needed in patients with mild hepatic impairment [see Warnings and Precautions ( 5.5) and Clinical Pharmacology ( 12.3)] .
8.7 Renal Impairment
No dosage adjustment is needed in patients with renal impairment [see Clinical Pharmacology ( 12.3)] .
Patients with End-Stage Renal Disease on dialysis
Clinical efficacy and safety studies with ticagrelor did not enroll patients with end-stage renal disease (ESRD) on dialysis. In patients with ESRD maintained on intermittent hemodialysis, no clinically significant difference in concentrations of ticagrelor and its metabolite and platelet inhibition are expected compared to those observed in patients with normal renal function [see Clinical Pharmacology ( 12.3)] . It is not known whether these concentrations will lead to similar efficacy and safety in patients with ESRD on dialysis as were seen in THEMIS and THALES.
-
10 OVERDOSAGE
There is currently no known treatment to reverse the effects of ticagrelor, and ticagrelor is not dialyzable. Treatment of overdose should follow local standard medical practice. Bleeding is the expected pharmacologic effect of overdosing. If bleeding occurs, appropriate supportive measures should be taken.
Platelet transfusion did not reverse the antiplatelet effect of ticagrelor in healthy volunteers and is unlikely to be of clinical benefit in patients with bleeding.
Other effects of overdose may include gastrointestinal effects (nausea, vomiting, diarrhea) or ventricular pauses. Monitor the ECG.
-
11 DESCRIPTION
Ticagrelor tablets contain ticagrelor, a cyclopentyltriazolopyrimidine, inhibitor of platelet activation and aggregation mediated by the P2Y 12 ADP-receptor. Chemically it is (1 S,2 S,3 R,5 S)-3-[7-{[(1 R,2 S)-2-(3,4-difluorophenyl)cyclopropyl]amino}-5-(propylthio)-3 H-[1,2,3]-triazolo[4,5- d]pyrimidin-3-yl]-5-(2-hydroxyethoxy)cyclopentane-1,2-diol. The empirical formula of ticagrelor is C 23H 28F 2N 6O 4S and its molecular weight is 522.57. The chemical structure of ticagrelor is:
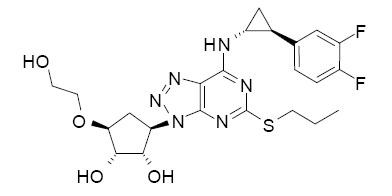
Ticagrelor is a crystalline powder with an aqueous solubility of approximately 10 μg/mL at room temperature.
Ticagrelor 90 mg tablets for oral administration contain 90 mg of ticagrelor and the following ingredients: microcrystalline cellulose, pregelatinized starch, croscarmellose sodium, hypromellose E5, polyethylene glycol 4000, colloidal silicon dioxide, magnesium stearate, hypromellose 2910, titanium dioxide, polyethylene glycol 400, ferric oxide yellow, ferric oxide red, and ferrosoferric oxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Ticagrelor and its major metabolite reversibly interact with the platelet P2Y 12 ADP-receptor to prevent signal transduction and platelet activation. Ticagrelor and its active metabolite are approximately equipotent.
12.2 Pharmacodynamics
The inhibition of platelet aggregation (IPA) by ticagrelor and clopidogrel was compared in a 6-week study examining both acute and chronic platelet inhibition effects in response to 20 μM ADP as the platelet aggregation agonist.
The onset of IPA was evaluated on Day 1 of the study following loading doses of 180 mg ticagrelor or 600 mg clopidogrel. As shown in Figure 5, IPA was higher in the ticagrelor group at all time points. The maximum IPA effect of ticagrelor was reached at around 2 hours, and was maintained for at least 8 hours.
The offset of IPA was examined after 6 weeks on ticagrelor 90 mg twice daily or clopidogrel 75 mg daily, again in response to 20 μM ADP.
As shown in Figure 6, mean maximum IPA following the last dose of ticagrelor was 88% and 62% for clopidogrel. The insert in Figure 6 shows that after 24 hours, IPA in the ticagrelor group (58%) was similar to IPA in clopidogrel group (52%), indicating that patients who miss a dose of ticagrelor would still maintain IPA similar to the trough IPA of patients treated with clopidogrel. After 5 days, IPA in the ticagrelor group was similar to IPA in the placebo group. It is not known how either bleeding risk or thrombotic risk track with IPA, for either ticagrelor or clopidogrel.Figure 5 - Mean inhibition of platelet aggregation (±SE) following single oral doses of placebo, 180 mg ticagrelor or 600 mg clopidogrel
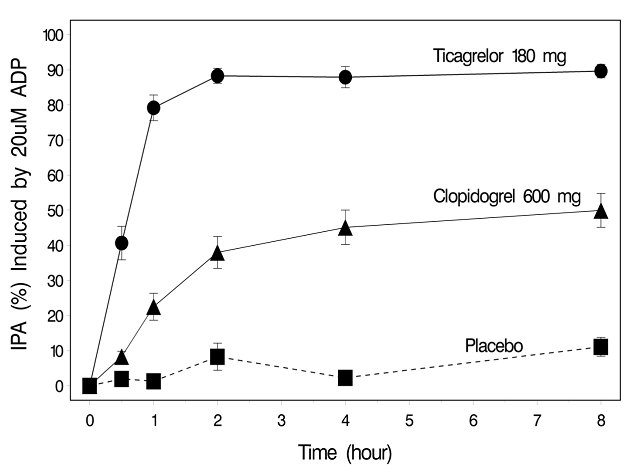
Figure 6 - Mean inhibition of platelet aggregation (IPA) following 6 weeks on placebo, ticagrelor 90 mg twice daily, or clopidogrel 75 mg daily
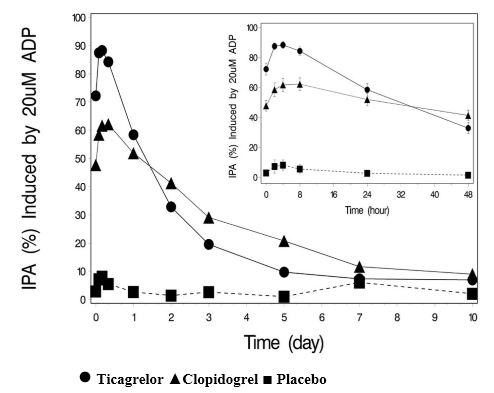
Ticagrelor ▲ Clopidogrel ■ Placebo
Transitioning from clopidogrel to ticagrelor tablets resulted in an absolute IPA increase of 26.4% and from ticagrelor tablets to clopidogrel resulted in an absolute IPA decrease of 24.5%. Patients can be transitioned from clopidogrel to ticagrelor tablets without interruption of antiplatelet effect [see Dosage and Administration (2)].
12.3 Pharmacokinetics
Ticagrelor demonstrates dose proportional pharmacokinetics, which are similar in patients and healthy volunteers.
AbsorptionTicagrelor can be taken with or without food. Absorption of ticagrelor occurs with a median t max of 1.5 h (range 1.0-4.0). The formation of the major circulating metabolite AR-C124910XX (active) from ticagrelor occurs with a median t max of 2.5 h (range 1.5-5.0).
The mean absolute bioavailability of ticagrelor is about 36% (range 30%-42%). Ingestion of a high-fat meal had no effect on ticagrelor C max, but resulted in a 21% increase in AUC. The C max of its major metabolite was decreased by 22% with no change in AUC.
Ticagrelor as crushed tablets mixed in water, given orally or administered through a nasogastric tube into the stomach, is bioequivalent to whole tablets (AUC and C max within 80-125% for ticagrelor and AR-C124910XX) with a median t max of 1.0 hour (range 1.0-4.0) for ticagrelor and 2.0 hours (range 1.0-8.0) for AR-C124910XX.
Distribution
The steady state volume of distribution of ticagrelor is 88 L. Ticagrelor and the active metabolite are extensively bound to human plasma proteins (>99%).Metabolism
CYP3A4 is the major enzyme responsible for ticagrelor metabolism and the formation of its major active metabolite. Ticagrelor and its major active metabolite are weak P-glycoprotein substrates and inhibitors. The systemic exposure to the active metabolite is approximately 30-40% of the exposure of ticagrelor. Ticagrelor is a BCRP inhibitor.
Excretion
The primary route of ticagrelor elimination is hepatic metabolism. When radiolabeled ticagrelor is administered, the mean recovery of radioactivity is approximately 84% (58% in feces, 26% in urine). Recoveries of ticagrelor and the active metabolite in urine were both less than 1% of the dose. The primary route of elimination for the major metabolite of ticagrelor is most likely to be biliary secretion. The mean t 1/2 is approximately 7 hours for ticagrelor and 9 hours for the active metabolite.
Specific Populations
The effects of age, gender, ethnicity, renal impairment and mild hepatic impairment on the pharmacokinetics of ticagrelor are presented in Figure 7. Effects are modest and do not require dose adjustment.
Patients with End-Stage Renal Disease on Hemodialysis
In patients with end stage renal disease on hemodialysis AUC and C max of ticagrelor 90 mg administered on a day without dialysis were 38% and 51% higher respectively, compared to subjects with normal renal function. A similar increase in exposure was observed when ticagrelor was administered immediately prior to dialysis showing that ticagrelor is not dialyzable. Exposure of the active metabolite increased to a lesser extent. The IPA effect of ticagrelor was independent of dialysis in patients with end stage renal disease and similar to healthy adults with normal renal function.
Figure 7 - Impact of intrinsic factors on the pharmacokinetics of ticagrelor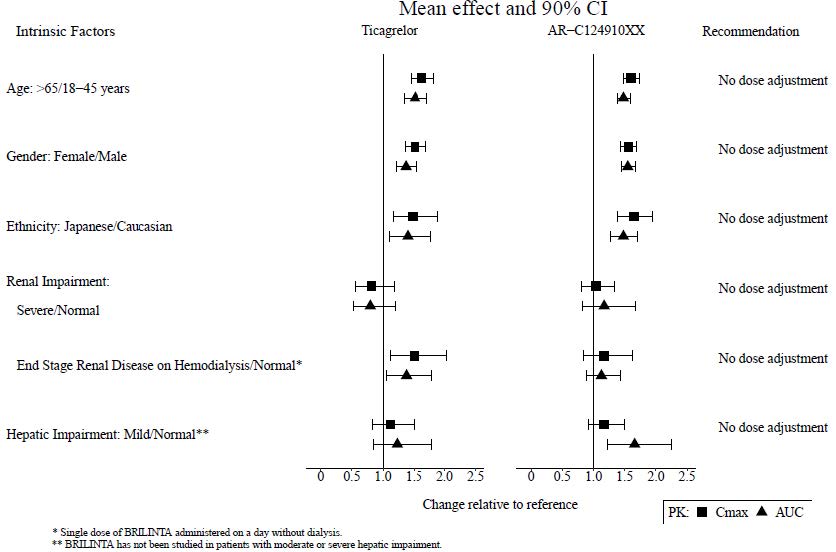
Effects of Other Drugs on Ticagrelor Tablets
CYP3A4 is the major enzyme responsible for ticagrelor metabolism and the formation of its major active metabolite. The effects of other drugs on the pharmacokinetics of ticagrelor are presented in Figure 8 as change relative to ticagrelor given alone (test/reference). Strong CYP3A inhibitors (e.g., ketoconazole, itraconazole, and clarithromycin) substantially increase ticagrelor exposure. Moderate CYP3A inhibitors have lesser effects (e.g., diltiazem). CYP3A inducers (e.g., rifampin) substantially reduce ticagrelor blood levels. P-gp inhibitors (e.g., cyclosporine) increase ticagrelor exposure.
Co-administration of 5 mg intravenous morphine with 180 mg loading dose of ticagrelor decreased observed mean ticagrelor exposure by up to 25% in healthy adults and up to 36% in ACS patients undergoing PCI. T max was delayed by 1-2 hours. Exposure of the active metabolite decreased to a similar extent. Morphine co-administration did not delay or decrease platelet inhibition in healthy adults. Mean platelet aggregation was higher up to 3 hours post loading dose in ACS patients co-administered with morphine.
Co-administration of intravenous fentanyl with 180 mg loading dose of ticagrelor in ACS patients undergoing PCI resulted in similar effects on ticagrelor exposure and platelet inhibition.Figure 8 - Effect of co-administered drugs on the pharmacokinetics of ticagrelor
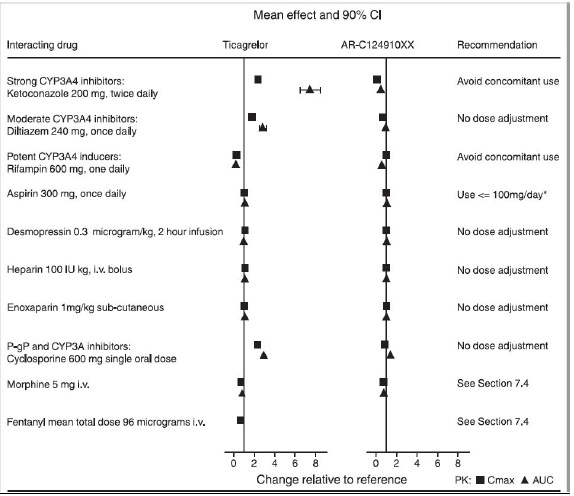
*See Dosage and Administration ( 2).
Effects of Ticagrelor Tablets on Other DrugsIn vitro metabolism studies demonstrate that ticagrelor and its major active metabolite are weak inhibitors of CYP3A4, potential activators of CYP3A5 and inhibitors of the P-gp transporter. In vitro metabolism studies demonstrate that ticagrelor is a BCRP inhibitor. Ticagrelor and AR-C124910XX were shown to have no inhibitory effect on human CYP1A2, CYP2C19, and CYP2E1 activity. For specific in vivo effects on the pharmacokinetics of simvastatin, atorvastatin, ethinyl estradiol, levonorgesterol, tolbutamide, digoxin and cyclosporine, see Figure 9.
Figure 9 - Impact of ticagrelor tablets on the pharmacokinetics of co-administered drugs
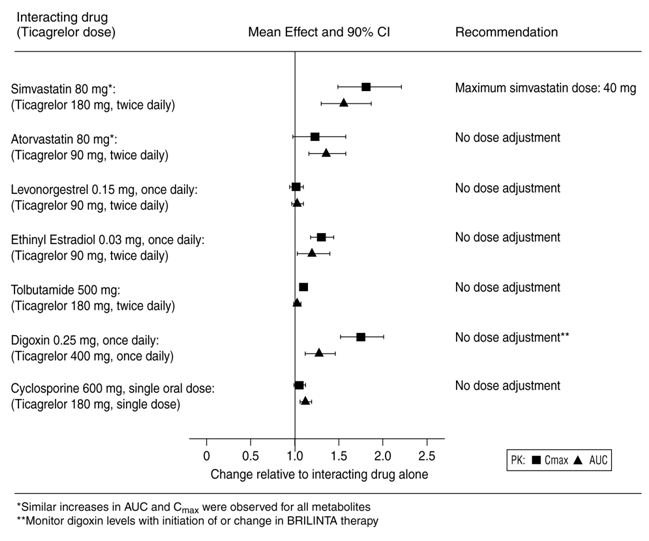
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Ticagrelor was not carcinogenic in the mouse at doses up to 250 mg/kg/day or in the male rat at doses up to 120 mg/kg/day (19 and 15 times the MRHD of 90 mg twice daily on the basis of AUC, respectively). Uterine carcinomas, uterine adenocarcinomas and hepatocellular adenomas were seen in female rats at doses of 180 mg/kg/day (29-fold the maximally recommended dose of 90 mg twice daily on the basis of AUC), whereas 60 mg/kg/day (8-fold the MRHD based on AUC) was not carcinogenic in female rats.
Mutagenesis
Ticagrelor did not demonstrate genotoxicity when tested in the Ames bacterial mutagenicity test, mouse lymphoma assay and the rat micronucleus test. The active O-demethylated metabolite did not demonstrate genotoxicity in the Ames assay and mouse lymphoma assay.
Impairment of Fertility
Ticagrelor had no effect on male fertility at doses up to 180 mg/kg/day or on female fertility at doses up to 200 mg/kg/day (>15-fold the MRHD on the basis of AUC). Doses of ≥10 mg/kg/day given to female rats caused an increased incidence of irregular duration estrus cycles (1.5-fold the MRHD based on AUC).
-
14 CLINICAL STUDIES
14.2 Coronary Artery Disease but No Prior Stroke or Myocardial Infarction
THEMIS
The THEMIS study (NCT01991795) was a double-blind, parallel group, study in which 19,220 patients with CAD and Type 2 Diabetes Mellitus (T2DM) but no history of MI or stroke were randomized to twice daily ticagrelor tablets or placebo, on a background of 75-150 mg of aspirin. The primary endpoint was the composite of first occurrence of CV death, MI, and stroke. CV death, MI, ischemic stroke, and all-cause death were assessed as secondary endpoints.
Patients were eligible to participate if they were ≥ 50 years old with CAD, defined as a history of PCI or CABG, or angiographic evidence of ≥ 50% lumen stenosis of at least 1 coronary artery and T2DM treated for at least 6 months with glucose-lowering medication. Patients with previous intracerebral hemorrhage, gastrointestinal bleeding within the past 6 months, known bleeding diathesis, and coagulation disorder were excluded. Patients taking anticoagulants or ADP receptor antagonists were excluded from participating, and patients who developed an indication for those medications during the trial were discontinued from study drug.
Patients were treated for a median of 33 months and up to 58 months.
Patients were predominantly male (69%) with a mean age of 66 years. At baseline, 80% had a history of coronary artery revascularization; 58% had undergone PCI, 29% had undergone a CABG and 7% had undergone both. The proportion of patients studied in the US was 12%. Patients in THEMIS had established CAD and other risk factors that put them at higher cardiovascular risk.
Ticagrelor tablets were superior to placebo in reducing the incidence of CV death, MI, or stroke. The effect on the composite endpoint was driven by the individual components MI and stroke; see Table 9.Table 9 – Primary composite endpoint, primary endpoint components, and secondary endpoints (THEMIS)
Ticagrelor Tablets
N=9619Placebo
N=9601HR (95% CI) p-value Events / 1000 patient years Events / 1000 patient years Time to first CV death, MI, or stroke * 24 27 0.90 (0.81, 0.99) 0.04 CV death † 12 11 1.02 (0.88, 1.18) Myocardial infarction † 9 11 0.84 (0.71, 0.98) Stroke † 6 7 0.82 (0.67, 0.99) Secondary endpoints CV death 12 11 1.02 (0.88, 1.18) Myocardial infarction 9 11 0.84 (0.71, 0.98) Ischemic stroke 5 6 0.80 (0.64, 0.99) All-cause death 18 19 0.98 (0.87, 1.10) CI = Confidence interval; CV = Cardiovascular; HR = Hazard ratio; MI = Myocardial infarction.
* Primary endpoint
† The event rate for the components CV death, MI and stroke are calculated from the actual number of first events for each component.The Kaplan-Meier curve (Figure 15) shows time to first occurrence of the primary composite endpoint of CV death, MI, or stroke.
Figure 15 - Time to First Occurrence of CV death, MI or Stroke (THEMIS)
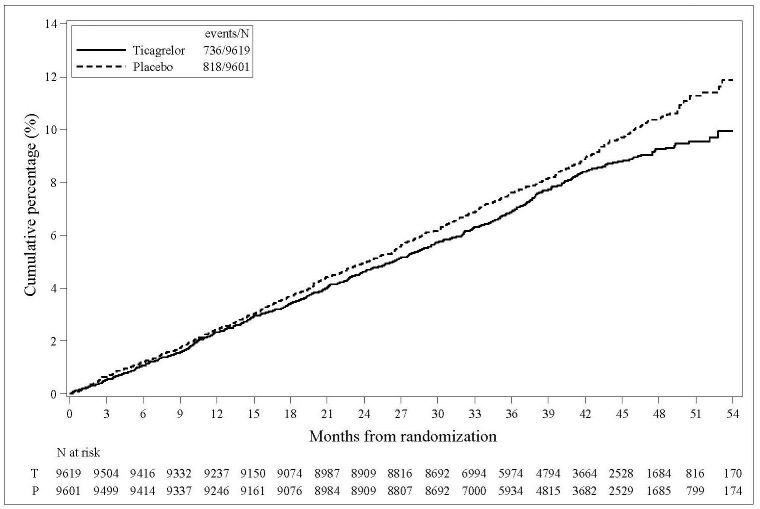
T = Ticagrelor; P = Placebo; N = Number of patients.
The treatment effect of ticagrelor tablets appeared similar across patient subgroups, see Figure 16.Figure 16 –Subgroup analyses of ticagrelor (THEMIS)
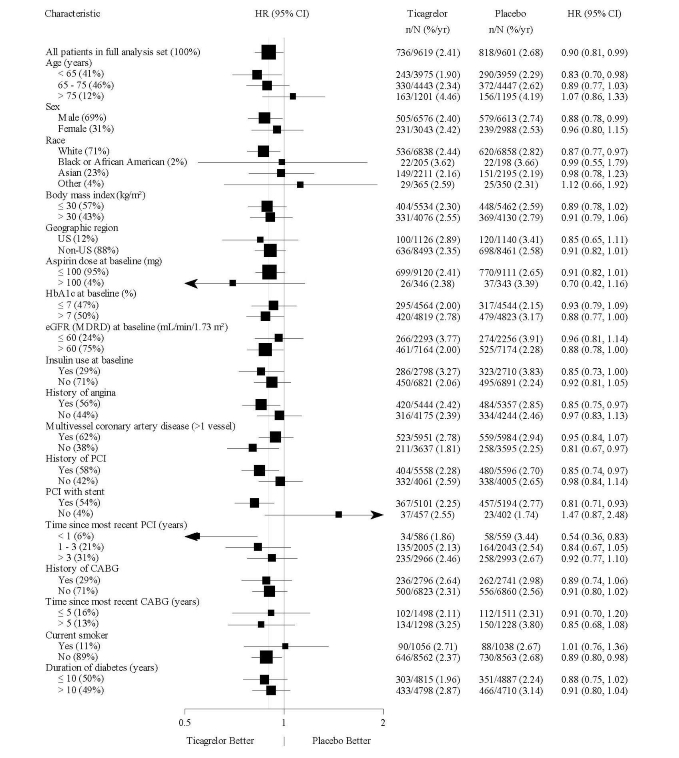
Note: The figure above presents effects in various subgroups all of which are baseline characteristics. The 95% confidence limits that are shown do not take into account how many comparisons were made, nor do they reflect the effect of a particular factor after adjustment for all other factors.
Apparent homogeneity or heterogeneity among groups should not be over-interpreted.14.3 Acute Ischemic Stroke or Transient Ischemic Attack (TIA)
THALES
The THALES study (NCT03354429) was a 11,016-patient, randomized, double-blind, parallel-group study of ticagrelor 90 mg twice daily versus placebo in patients with acute ischemic stroke or transient ischemic attack (TIA). The primary endpoint was the first occurrence of the composite of stroke and death up to 30 days. Ischemic stroke was assessed as one of the secondary endpoints.Patients were eligible to participate if they were ≥40 years old, with non-cardioembolic acute ischemic stroke (NIHSS score ≤5) or high-risk TIA (defined as ABCD 2 score ≥6 or ipsilateral atherosclerotic stenosis ≥50% in the internal carotid or an intracranial artery). Patients who received thrombolysis or thrombectomy within 24 hours prior to randomization were not eligible.
Patients were randomized within 24 hours of onset of an acute ischemic stroke or TIA to receive 30 days of either ticagrelor tablets (90 mg twice daily, with an initial loading dose of 180 mg) or placebo, on a background of aspirin initially 300-325 mg then 75-100 mg daily. The median treatment duration was 31 days.Ticagrelor was superior to placebo in reducing the rate of the primary endpoint (composite of stroke and death), corresponding to a relative risk reduction (RRR) of 17% and an absolute risk reduction (ARR) of 1.1% (Table 10). The effect was driven primarily by a significant reduction in the stroke component of the primary endpoint (19% RRR, 1.1% ARR).
Table 10 - Incidences of the primary composite endpoint, primary composite endpoint components, and secondary endpoint (THALES)Ticagrelor Tablets
N=5523Placebo
N=5493HR (95% CI) p-value n (patients with event) KM% n (patients with event) KM% Time to first Stroke or Death 303 5.4% 362 6.5% 0.83 (0.71, 0.96) 0.015 Time to first Stroke * 284 5.1% 347 6.3% 0.81 (0.69, 0.95) Time to Death * 36 0.6% 27 0.5% 1.33 (0.81, 2.19) Secondary Endpoint Time to first Ischemic Stroke 276 5.0% 345 6.2% 0.79 (0.68, 0.93) 0.04 CI = Confidence interval; HR = Hazard ratio; KM = Kaplan-Meier percentage calculated at 30 days; N = Number of patients
* The number of patients with the event of interest. In the time to first stroke, patients who died are censored at the time of death.
The Kaplan-Meier curve (Figure 17) shows the time to first occurrence of the primary composite endpoint of stroke and death.Figure 17 – Time to First Occurrence of Stroke or Death (THALES)
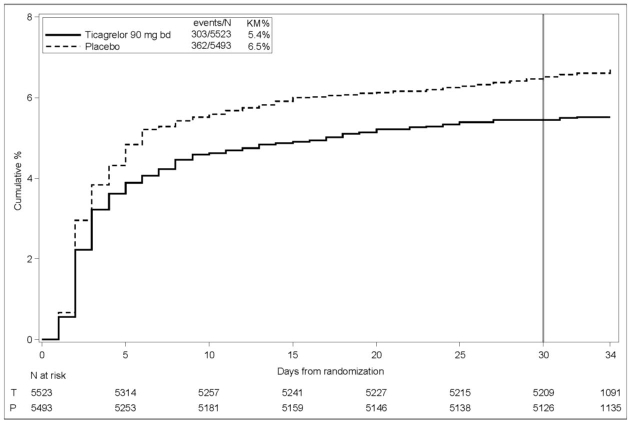
KM%: Kaplan-Meier percentage evaluated at Day 30; T=Ticagrelor; P=placebo; N=Number of patients
Ticagrelor tablets’ treatment effect on stroke and on death accrued over the first 10 days and was sustained at 30 days. Although not studied, this suggests that shorter treatment could result in similar benefit and reduced bleeding risk.
The treatment effect of ticagrelor tablets was generally consistent across pre-defined subgroups (Figure 18).Figure 18 – Subgroup analyses of ticagrelor 90 mg (THALES)
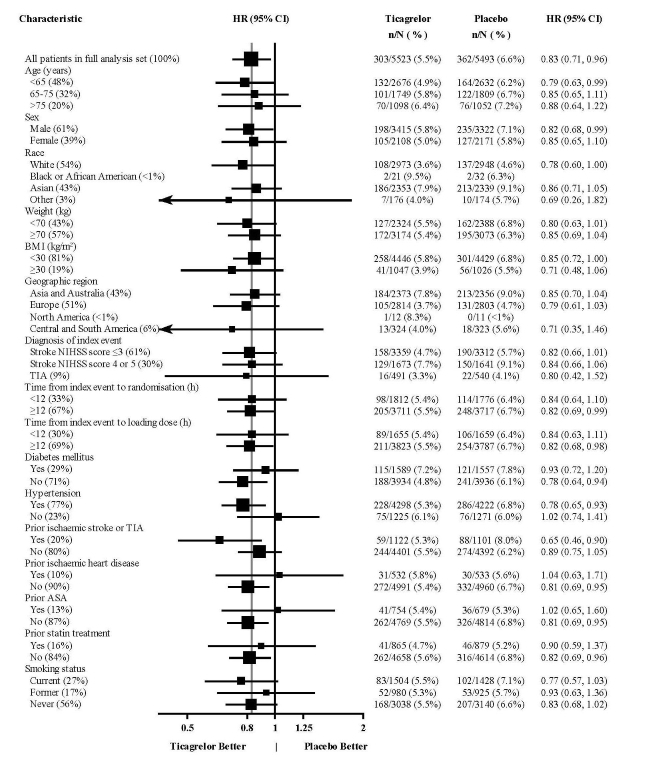
Note: The figure above presents effects in various subgroups all of which are baseline characteristics and were pre-specified. The 95% confidence limits that are shown do not take into account how many comparisons were made, nor do they reflect the effect of a particular factor after adjustment for all other factors. Apparent homogeneity or heterogeneity among groups should not be over-interpreted.
At Day 30, there was an absolute reduction of 1.2% (95% CI: -2.1%, -0.3%) in the incidence of non-hemorrhagic stroke and death (excluding fatal bleed) favoring ticagrelor (294 events: 5.3%) over placebo (359 events: 6.5%) in the intention-to-treat population. In the same population, there was an absolute increase of 0.4% (95% CI: 0.2%, 0.6%) in the incidence of GUSTO severe bleeding unfavorable to ticagrelor arm (28 events: 0.5%) compared to the placebo arm (7 events: 0.1%). -
16 HOW SUPPLIED/STORAGE AND HANDLING
Ticagrelor tablets 90 mg are supplied as a round, biconvex, yellow, film-coated tablet debossed with “HU” on one side and “90” on the other side.
Bottles of 60 NDC: 42658-115-03
Bottles of 180 NDC: 42658-115-10
Bottles of 500 NDC: 42658-115-07Storage and Handling
Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [See USP Controlled Room Temperature].
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide)
Advise patients daily doses of aspirin should not exceed 100 mg and to avoid taking any other medications that contain aspirin.
Advise patients that they:
- Will bleed and bruise more easily
- Will take longer than usual to stop bleeding
- Should report any unanticipated, prolonged or excessive bleeding, or blood in their stool or urine.
Advise patients to contact their doctor if they experience unexpected shortness of breath, especially if severe.
Advise patients to inform physicians and dentists that they are taking ticagrelor tablets before any surgery or dental procedure.
Advise women that breastfeeding is not recommended during treatment with ticagrelor [see Use in Specific Populations ( 8.2)].
Distributed by:
Hisun Pharmaceuticals USA, Inc.
Bridgewater, NJ 08807
Manufactured in China.
Revised: 11/2024
-
MEDICATION GUIDE
TICAGRELOR (tye-KA-grel-or) Tablets
What is the most important information I should know about ticagrelor tablets?
Ticagrelor tablets are used to lower your chance of having, or dying from, a heart attack or stroke. Ticagrelor tablets (and similar drugs) can cause bleeding that can be serious and sometimes lead to death. In cases of serious bleeding, such as internal bleeding, the bleeding may result in the need for blood transfusions or surgery. While you take ticagrelor tablets:
you may bruise and bleed more easily
you are more likely to have nose bleeds
it will take longer than usual for any bleeding to stop
Call your healthcare provider right away, if you have any of these signs or symptoms of bleeding while taking ticagrelor tablets:
bleeding that is severe or that you cannot control
pink, red or brown urine
vomiting blood or your vomit looks like “coffee grounds”
red or black stools (looks like tar)
coughing up blood or blood clots
Do not stop taking ticagrelor tablets without talking to the healthcare provider who prescribes it for you. People who are treated with a stent, and stop taking ticagrelor tablets too soon, have a higher risk of getting a blood clot in the stent, having a heart attack, or dying. If you stop ticagrelor tablets because of bleeding, or for other reasons, your risk of a heart attack or stroke may increase.
Your healthcare provider may instruct you to stop taking ticagrelor tablets 5 days before surgery. This will help to decrease your risk of bleeding with your surgery or procedure. Your healthcare provider should tell you when to start taking ticagrelor tablets again, as soon as possible after surgery.
Taking ticagrelor tablets with aspirin
Ticagrelor tablets are taken with aspirin, unless your healthcare provider specifically tells you otherwise. Talk to your healthcare provider about the dose of aspirin that you should take with ticagrelor tablets. In most cases, you should not take a dose of aspirin higher than 100 mg daily. Do not take doses of aspirin higher than what your healthcare provider tells you to take. Tell your healthcare provider if you take other medicines that contain aspirin, and do not take new over-the-counter medicines with aspirin in them.
What are ticagrelor tablets?
Ticagrelor tablets are a prescription medicine used to:
decrease your risk of a first heart attack or stroke in people who have a condition where the blood flow to the heart is decreased (coronary artery disease or CAD) who are at high risk for having a heart attack or stroke.
decrease your risk of stroke in people who are having a stroke (acute ischemic stroke) or mini-stroke (transient ischemic attack or TIA).
It is not known if ticagrelor tablets are safe and effective in children.
Do not take ticagrelor tablets if you:
have a history of bleeding in the brain
are bleeding now
are allergic to ticagrelor or any of the ingredients in ticagrelor tablets. See the end of this Medication Guide for a complete list of ingredients in ticagrelor tablets.
Before taking ticagrelor tablets, tell your healthcare provider about all of your medical conditions, if you:
have had bleeding problems in the past
have had any recent serious injury or surgery
plan to have surgery or a dental procedure. See “ What is the most important information I should know about ticagrelor tablets?”
have a history of stomach ulcers or colon polyps
have lung or breathing problems, such as COPD or asthma
have liver problems
have a history of stroke
are pregnant or plan to become pregnant. It is not known if ticagrelor tablets will harm your unborn baby. You and your healthcare provider should decide if you will take ticagrelor tablets.
are breastfeeding or plan to breastfeed. It is not known if ticagrelor passes into your breast milk. You should not breastfeed during treatment with ticagrelor tablets. Talk to your healthcare provider about the best way to feed your baby during treatment with ticagrelor tablets.
Tell all of your healthcare providers and dentists that you are taking ticagrelor tablets. They should talk to the healthcare provider who prescribed ticagrelor tablets for you before you have any surgery or procedure.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter
medicines, vitamins, and herbal supplements. Ticagrelor tablets may affect the way other medicines work, and other medicines may affect how ticagrelor tablets work. Certain medicines may increase your risk of bleeding.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take ticagrelor tablets?
Take ticagrelor tablets exactly as prescribed by your healthcare provider.
Your healthcare provider will tell you how many ticagrelor tablets to take and when to take them.
Take ticagrelor tablets with aspirin, unless your healthcare provider specifically tells you otherwise. See “ What is the most important information I should know about ticagrelor tablets?”
You may take ticagrelor tablets with or without food.
Take ticagrelor tablets two times each day, around the same times each day.
If you miss your scheduled dose of ticagrelor tablets, take your next dose at its scheduled time. Do not take 2 doses at the same time unless your healthcare provider tells you to.
If you take too much ticagrelor tablets, call your healthcare provider or local poison control center or go to the nearest emergency room right away.
If you are unable to swallow the tablet(s) whole, you may crush the ticagrelor tablet(s) and mix it with water. Drink all the water right away. Refill the glass with water, stir, and drink all the water.
Ticagrelor tablets may also be given through certain nasogastric (NG) tubes. Ask your healthcare provider for instructions on how to take ticagrelor tablets through a NG tube.
What are the possible side effects of ticagrelor tablets?
Ticagrelor tablets can cause serious side effects, including:
See “What is the most important information I should know about ticagrelor tablets?”
Shortness of breath. Tell your healthcare provider if you have new, worsening or unexpected shortness of breath when you are at rest, at night, or when you are doing any activity.
Slow or irregular heartbeat.
Irregular breathing. Tell your healthcare provider if you develop irregular breathing patterns when asleep or awake such as speeding up, slowing down or short pauses in breathing. Your healthcare provider will decide if you need further evaluation.
These are not all of the possible side effects of ticagrelor tablets.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800- FDA-1088.
How should I store ticagrelor tablets?
Store ticagrelor tablets at room temperature between 68°F to 77°F (20°C to 25°C).
Keep ticagrelor tablets and all medicines out of the reach of children.
General information about the safe and effective use of ticagrelor tablets
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use ticagrelor tablets for a condition for which it was not prescribed. Do not give ticagrelor tablets to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about ticagrelor tablets that is written for health professionals.
What are the ingredients in ticagrelor tablets?
Active ingredient: ticagrelor
Inactive ingredients: microcrystalline cellulose, pregelatinized starch, croscarmellose sodium, hypromellose E5, polyethylene glycol 4000, colloidal silicon dioxide, magnesium stearate, hypromellose 2910, titanium dioxide, polyethylene glycol 400, ferric oxide yellow, ferric oxide red, and ferrosoferric oxide.
Distributed by:
Hisun Pharmaceuticals USA, Inc.
Bridgewater, NJ 08807
Manufactured in China.
For more information call 1-855-554-4786 or go to www.hisunusa.com.
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 10/2024
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TICAGRELOR
ticagrelor tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 42658-115 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TICAGRELOR (UNII: GLH0314RVC) (TICAGRELOR - UNII:GLH0314RVC) TICAGRELOR 90 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) STARCH, PREGELATINIZED CORN (UNII: O8232NY3SJ) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSE 2910 (5 MPA.S) (UNII: R75537T0T4) POLYETHYLENE GLYCOL 4000 (UNII: 4R4HFI6D95) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) WATER (UNII: 059QF0KO0R) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Product Characteristics Color yellow Score no score Shape ROUND (biconvex) Size 10mm Flavor Imprint Code HU;90 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42658-115-03 60 in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2025 2 NDC: 42658-115-10 180 in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2025 3 NDC: 42658-115-07 500 in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA208575 01/01/2025 Labeler - Hisun Pharmaceuticals USA, Inc. (961628505) Registrant - Hisun Pharmaceutical (Hangzhou) Co., Ltd. (421250870) Establishment Name Address ID/FEI Business Operations Hanhui Pharmaceuticals Co., Ltd. 421307115 manufacture(42658-115)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
