BRIGHTEN LIGHTENING- hydroquinone gel
BRIGHTEN by
Drug Labeling and Warnings
BRIGHTEN by is a Otc medication manufactured, distributed, or labeled by CEN BEAUTY LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
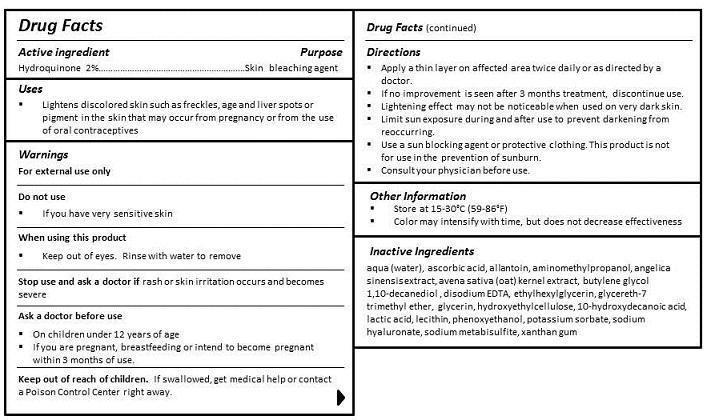
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- ASK DOCTOR
-
DOSAGE & ADMINISTRATION
DIRECTIONS
- Apply a thin layer on affected area twice daily or as directed by a doctor.
- If no improvement is seen after 3 months treatment, discontinue use.
- Lightening effect may not be noticeable when used on very dark skin.
- Limit sun exposure during and after use to prevent darkening from reoccurring.
- Use a sun blocking agent or protective clothing. This product is not for use in the prevention of sunburn.
- Consult your physician before use.
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
INGREDIENTS
aqua (water), cyclopentasiloxane, PEG-10 dimethicone, dimethicone, butylene glycol, cyclohexasiloxane, glycerin, carollina officinalis extract, algae extract, avena sativa (oat) kernel extract, angelica sinensis extract, 10-hydroxydecanoic acid, sebacic acid, 1,10-decanediol acid, betaine, dimethicone/vinyl dimethicone crosspolymer, lauryl PEG-9 polydimethylsiloxyethyl dimethicone, lecithin, dimethicone/PEG-10/15 crosspolymer, triethoxycaprylylsilane, decamethylcyclopentasiloxane, trifluoromethyl C1-C4 alkyl dimethicone, quaternium-90, bentonite, butylene glycol, sodium chloride, sodium citrate, ethylhexylglycerin, propylene carbonate, propylene glycol, potassium sorbate, methylisothiazolinone, iodopropynyl butylcarbamate, phenoxyethanol
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BRIGHTEN LIGHTENING
hydroquinone gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 54272-301 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROQUINONE (UNII: XV74C1N1AE) (HYDROQUINONE - UNII:XV74C1N1AE) HYDROQUINONE 2 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ASCORBIC ACID (UNII: PQ6CK8PD0R) ALLANTOIN (UNII: 344S277G0Z) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) GLYCERIN (UNII: PDC6A3C0OX) OAT (UNII: Z6J799EAJK) ANGELICA SINENSIS WHOLE (UNII: 697D19QDBN) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) 10-HYDROXYDECANOIC ACID (UNII: NP03XO416B) 1,10-DECANEDIOL (UNII: 5I577UDK52) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) EDETATE DISODIUM (UNII: 7FLD91C86K) LACTIC ACID (UNII: 33X04XA5AT) GLYCERETH-7 TRIMETHYL ETHER (UNII: XMC7402M60) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HYDROXYETHYL CELLULOSE (100 MPA.S AT 2%) (UNII: R33S7TK2EP) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SODIUM METABISULFITE (UNII: 4VON5FNS3C) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XANTHAN GUM (UNII: TTV12P4NEE) PHENOXYETHANOL (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54272-301-11 30 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part358A 02/21/2013 Labeler - CEN BEAUTY LLC (078664118) Registrant - CEN BEAUTY LLC (078664118)
Trademark Results [BRIGHTEN]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BRIGHTEN 98411710 not registered Live/Pending |
Alcon Inc. 2024-02-20 |
 BRIGHTEN 88082446 not registered Live/Pending |
TXU Energy Retail Company LLC 2018-08-17 |
 BRIGHTEN 86411109 5013510 Live/Registered |
GUANGZHOU BINFEN GARDENING CO.,LTD. 2014-10-01 |
 BRIGHTEN 86114386 4792793 Live/Registered |
Brighten Labs, LLC 2013-11-08 |
 BRIGHTEN 85067189 not registered Dead/Abandoned |
FUJIAN BRIGHTEN COLOR FLAME CO., LTD. 2010-06-20 |
 BRIGHTEN 85021755 4050144 Dead/Cancelled |
TXU Energy Retail Company LLC 2010-04-23 |
 BRIGHTEN 78614038 3210242 Dead/Cancelled |
Brighten Corporation 2005-04-21 |
 BRIGHTEN 73632428 1447689 Dead/Cancelled |
BRIGHTEN MACHINERY CO., LTD. 1986-11-25 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.