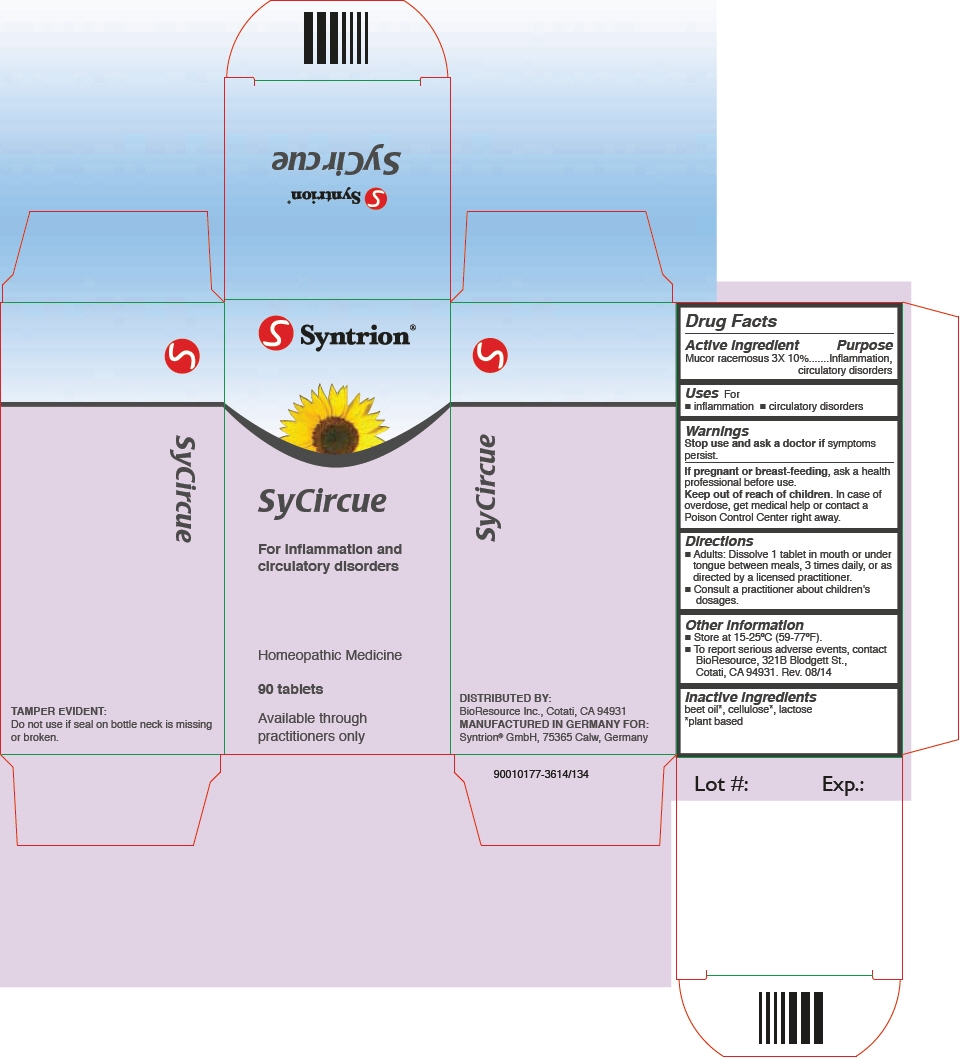
SyCircue by Syntrion GmbH SyCircue
SyCircue by
Drug Labeling and Warnings
SyCircue by is a Homeopathic medication manufactured, distributed, or labeled by Syntrion GmbH. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SYCIRCUE- mucor racemosus tablet
Syntrion GmbH
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
SyCircue
Directions
- Adults: Dissolve 1 tablet in mouth or under tongue between meals, 3 times daily, or as directed by a licensed practitioner.
- Consult a practitioner about children's dosages.
| SYCIRCUE
mucor racemosus tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Syntrion GmbH (331883145) |
Revised: 1/2016
Document Id: 861e2c25-c73c-44fb-9e9d-9b3350686981
Set id: c504aebc-b439-4648-834f-1a0386bfb417
Version: 2
Effective Time: 20160126
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.