HILL COUNTRY ESSENTIALS- dimethicone lotion
Hill Country Essentials by
Drug Labeling and Warnings
Hill Country Essentials by is a Otc medication manufactured, distributed, or labeled by H.E.B, Fruit of the Earth, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
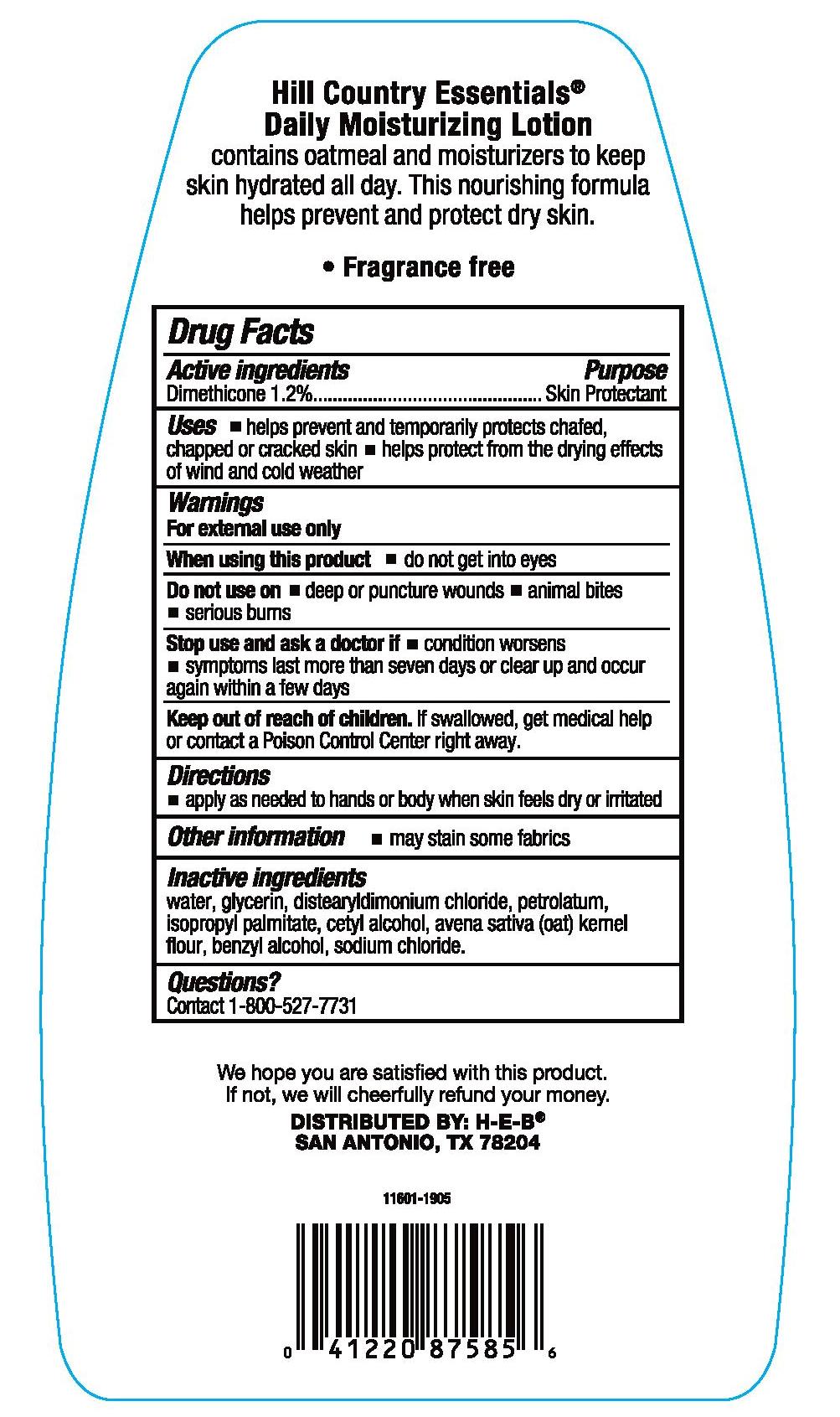
- Active ingredients
- Purpose
- Uses
- Warnings
- WHEN USING
- DO NOT USE
- ASK DOCTOR
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
PRINCIPAL DISPLAY PANEL
Hill Country Essentials Moisturizing Lotion
8 FL OZ (236mL)
NDC: 37808-995-69

Hill Country Essentials Moisturizing Lotion
18 FL OZ (532mL)
NDC: 37808-995-43

-
INGREDIENTS AND APPEARANCE
HILL COUNTRY ESSENTIALS
dimethicone lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 37808-995 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 12 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) DISTEARYLDIMONIUM CHLORIDE (UNII: OM9573ZX3X) PETROLATUM (UNII: 4T6H12BN9U) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) CETYL ALCOHOL (UNII: 936JST6JCN) OATMEAL (UNII: 8PI54V663Y) BENZYL ALCOHOL (UNII: LKG8494WBH) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 37808-995-69 236 mL in 1 TUBE; Type 0: Not a Combination Product 07/22/2010 2 NDC: 37808-995-43 532 mL in 1 BOTTLE; Type 0: Not a Combination Product 08/29/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 07/22/2010 Labeler - H.E.B (007924756) Registrant - Fruit of the Earth, Inc. (079559467) Establishment Name Address ID/FEI Business Operations Fruit of the Earth, Inc. 008193513 manufacture(37808-995)
Trademark Results [Hill Country Essentials]
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.











