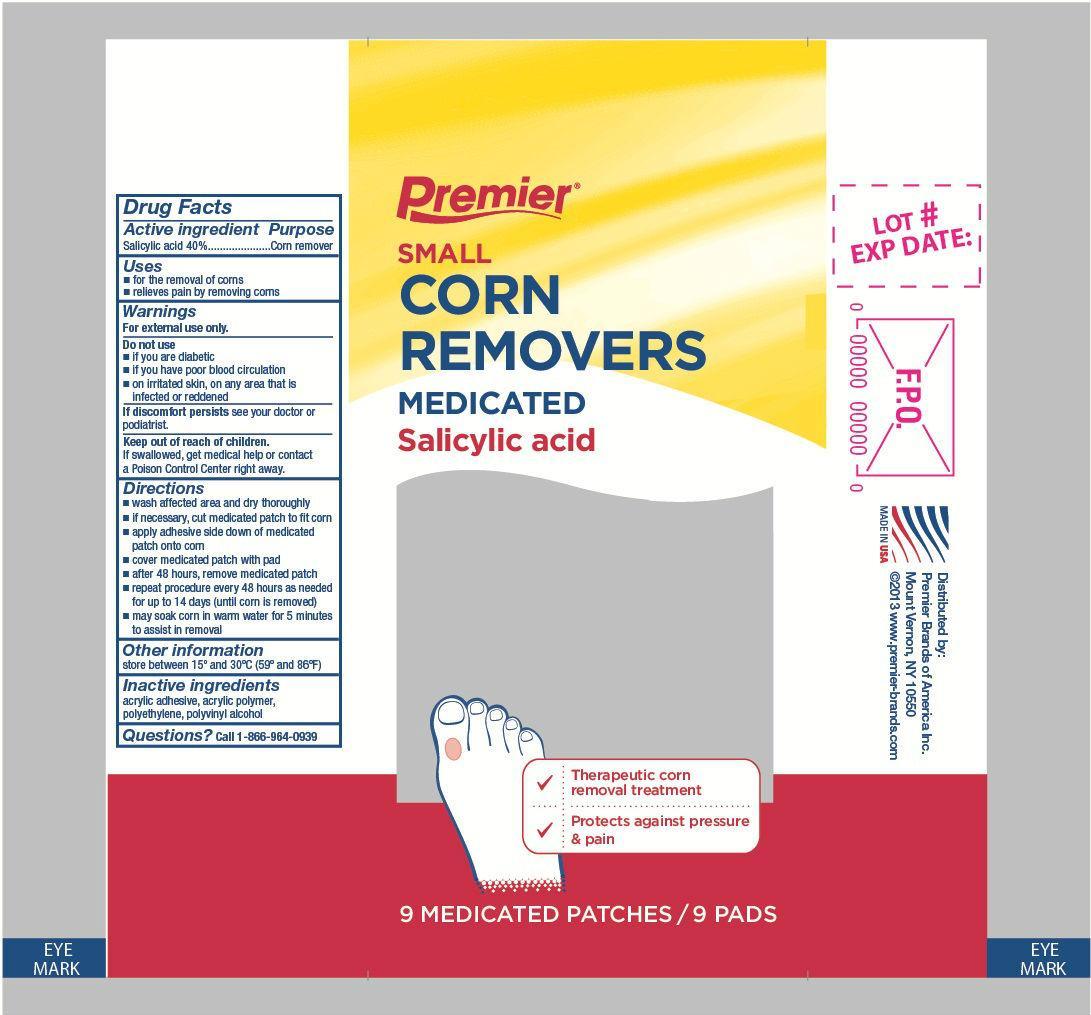
Premier Brands Small Corn Removers
Salicylic Acid by
Drug Labeling and Warnings
Salicylic Acid by is a Otc medication manufactured, distributed, or labeled by Premier Brands of America Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SALICYLIC ACID- small corn removers patch
Premier Brands of America Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Premier Brands Small Corn Removers
Warnings
For external use only.
Directions
- wash affected area and dry thoroughly
- if necessary, cut medicated patch to fit corn
- apply adhesive side down of medicated patch onto corn
- cover medicated patch with pad
- after 48 hours, remove medicated patch
- repeat procedure every 48 hours as needed for up to 14 days (until corn is removed)
- may soak corn in warm water for 5 minutes to assist in removal
| SALICYLIC ACID
small corn removers patch |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Premier Brands of America Inc. (080051232) |
Revised: 1/2020
Document Id: 9c587686-b8ba-3aad-e053-2a95a90af061
Set id: c52464bf-9ad4-4b23-aedd-e7b664620973
Version: 2
Effective Time: 20200117