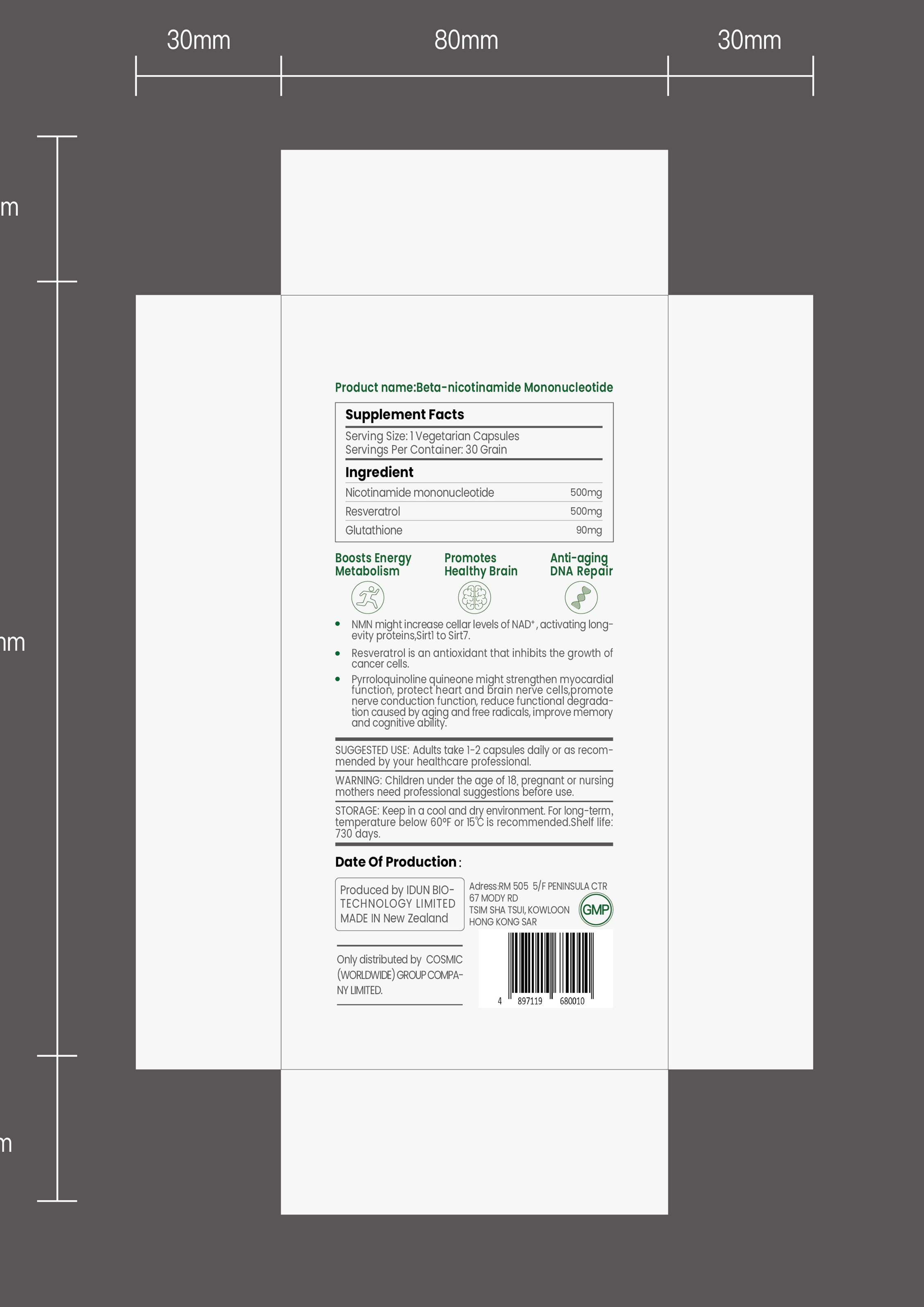
Nicotinamide mononucleotide(45.8%)
resveratrol(45.8%)
Children under the age of 18 need professional suggestions before use.
Boosts Energy Metabolism
Promotes Healthy Brain
Anti-aging DNA Repair
NMN might increase cellar levels of NAD+ , activating long-evity proteins,Sirtl to Sirt7.Resveratrol is an antioxidant that inhibits the growth ofcancer cells.
Pyrroloquinoline quineone might strengthen myocardial function, protect'heart and brain nerve cells,promotenerve conduction function, reduce functional degrada-tion caused by aging and free radicals, improve memoryand cognitive'ability.
SUGGESTED USE
Adults take 1-2 capsules daily or as recom-mended by your healthcare professional.
Keep in a cool and dry environment.For long-term,temperature below 60°F or 15℃ is recommended.
Children under the age of 18, pregnant or nursing mothers need professional suggestions before use.