Hydroquinone by TWi Pharmaceuticals, Inc. / Perrigo New York Inc HYDROQUINONE cream
Hydroquinone by
Drug Labeling and Warnings
Hydroquinone by is a Prescription medication manufactured, distributed, or labeled by TWi Pharmaceuticals, Inc., Perrigo New York Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
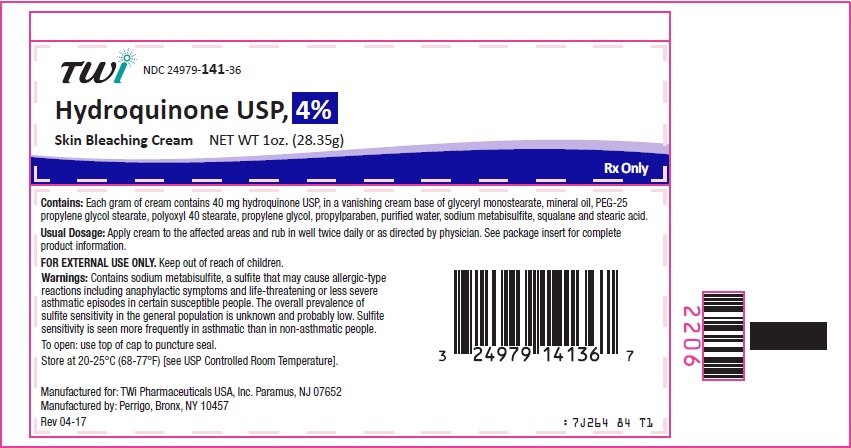
Each gram of Hydroquinone USP, 4% Skin Bleaching Cream contains 40 mg hydroquinone USP, in a vanishing cream base of glyceryl monostearate, mineral oil, PEG-25 propylene glycol stearate, polyoxyl 40 stearate, propylene glycol, propylparaben, purified water, sodium metabisulfite, squalane and stearic acid. Chemically, hydroquinone is C6H6O2 and has a molecular weight of 110.11.
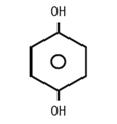
The chemical name is 1,4 dihydroxybenzene, and the structural formula of hydroquinone is:
-
CLINICAL PHARMACOLOGY
Topical application of hydroquinone produces a reversible depigmentation of the skin by inhibition of the enzymatic oxidation of tyrosine to 3,4-dihydroxyphenylalanine (dopa) (Denton, C. et al., 1952)1 and suppression of other melanocyte metabolic processes (Jimbow, K. et al., 1974)2. Exposure to sunlight or ultraviolet light will cause repigmentation of bleached areas (Parrish, J.A. et al., 1978)3.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
Contains sodium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in non-asthmatic people.
Since this product contains no sunscreen, an effective broad spectrum sun blocking agent should be used and unnecessary solar exposure avoided, or protective clothing should be worn to cover bleached skin in order to prevent repigmentation from occurring.
Hydroquinone may produce exogenous ochronosis, a gradual blue-black darkening of the skin. If this condition occurs, discontinue treatment and consult your physician. The majority of patients developing this condition are Black, but it may also occur in Caucasians and Hispanics.
-
PRECAUTIONS
(see WARNINGS)
General -
Test for skin sensitivity before using by applying a small amount to an unbroken patch of skin; check within 24 hours. Minor redness is not a contraindication, but where there is itching or vesicle formation or excessive inflammatory response further treatment is not advised. Close patient supervision is recommended.
Hydroquinone is a skin bleaching agent which may produce unwanted cosmetic effects if not used as directed. The physician should be familiar with the contents of this insert before prescribing or dispensing this medication.
Information for Patients -
Sunscreen use is an essential aspect of hydroquinone therapy because even minimal sunlight sustains melanocytic activity. To prevent repigmentation, during treatment and maintenance therapy, sun exposure on treated skin should be avoided by application of a broad spectrum sunscreen (SPF 15 or greater) or by use of protective clothing.
Avoid contact with eyes and mucous membranes.
Keep this and all medications out of reach of children. In case of accidental ingestion, call a physician or a poison control center immediately.
Drug Interactions -
Patients are cautioned on concomitant use of medications that are known to be photosensitizing.
Carcinogenesis, Mutagenesis, Impairment of Fertility -
Studies of hydroquinone in animals have demonstrated some evidence of carcinogenicity. The carcinogenic potential of hydroquinone in humans is unknown.
Published studies have demonstrated that hydroquinone is a mutagen and a clastogen. Treatment with hydroquinone has resulted in positive findings for genetic toxicity in the Ames assay in bacterial strains sensitive to oxidizing mutagens, in in vitro studies in mammalian cells, and in the in vivo mouse micronucleus assay.
Pregnancy:
Teratogenic Effects:
Pregnancy Category C -
Animal reproduction studies have not been conducted with topical hydroquinone. It is also not known whether topical hydroquinone can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Topical hydroquinone should be given to a pregnant woman only if clearly needed.
-
ADVERSE REACTIONS
The following adverse reactions have been reported: dryness and fissuring of paranasal and infraorbital areas, erythema, and stinging. Occasional hypersensitivity (localized contact dermatitis) may develop. If this occurs, the medication should be discontinued and the physician notified immediately.
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
Hydroquinone USP, 4% Skin Bleaching Cream should be applied to affected areas and rubbed in well twice daily, in the morning and before bedtime, or as directed by a physician. If no improvement is seen after 2 months of treatment, use of this product should be discontinued. There is no recommended dosage for pediatric patients under 12 years of age except under the advice and supervision of a physician.
- HOW SUPPLIED
- STORAGE AND HANDLING
-
SPL UNCLASSIFIED SECTION
REFERENCES
1 DENTON C., LERNER A.B., FITZPATRICK T.B.
Inhibition of Melanin Formation by Chemical Agents
Journal of Investigative Dermatology 1952, 18:119-135.
2 JIMBOW K., OBATA H., PATHAK M., FITZPATRICK T.B.
Mechanism of Depigmentation by Hydroquinone
Journal of Investigative Dermatology 1974, 62:436-449.
3 PARRISH J.A., ANDERSON R.R., URBACH F., PITTS D.
UVA, Biological Effects of Ultraviolet Radiation with Emphasis
on Human Responses to Longwave Ultraviolet
Plenum Press, New York and London, 1978, p. 151.
Manufactured for:
TWi pharmaceuticals USA, Inc.
Paramus, NJ 07652
Manufacured by:
Perrigo, Bronx, NY 10457
Rev 04-17
: 7J200 84 J1
- Principal Display Panel - 1 oz Carton
- Principal Display Panel - 1 oz Tube
-
INGREDIENTS AND APPEARANCE
HYDROQUINONE
hydroquinone creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 24979-141 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROQUINONE (UNII: XV74C1N1AE) (HYDROQUINONE - UNII:XV74C1N1AE) HYDROQUINONE 40 mg in 1 g Inactive Ingredients Ingredient Name Strength GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) MINERAL OIL (UNII: T5L8T28FGP) POLYOXYL 40 STEARATE (UNII: 13A4J4NH9I) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SODIUM METABISULFITE (UNII: 4VON5FNS3C) SQUALANE (UNII: GW89575KF9) STEARIC ACID (UNII: 4ELV7Z65AP) PEG-25 PROPYLENE GLYCOL STEARATE (UNII: X21KPH4633) Product Characteristics Color WHITE (white to off-white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 24979-141-36 1 in 1 CARTON 06/16/2017 1 28.35 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug other 06/16/2017 Labeler - TWi Pharmaceuticals, Inc. (658402052) Registrant - TWi Pharmaceuticals, Inc. (658402052) Establishment Name Address ID/FEI Business Operations Perrigo New York Inc 078846912 manufacture(24979-141) , analysis(24979-141) , pack(24979-141)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.