XIFAXAN- rifaximin tablet
XIFAXAN by
Drug Labeling and Warnings
XIFAXAN by is a Prescription medication manufactured, distributed, or labeled by Salix Pharmaceuticals, Inc., Valeant Pharmaceuticals North America LLC, Patheon Inc., Alfasigma SPA, Carton Service, Incorporated, Valeant Pharmaceuticals International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use XIFAXAN safely and effectively. See full prescribing information for XIFAXAN.
XIFAXAN® (rifaximin) tablets, for oral use
Initial U.S. Approval: 2004
To reduce the development of drug-resistant bacteria and maintain the effectiveness of XIFAXAN and other antibacterial drugs, XIFAXAN should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.INDICATIONS AND USAGE
XIFAXAN is a rifamycin antibacterial indicated for:
- Treatment of travelers’ diarrhea (TD) caused by noninvasive strains of Escherichia coli in adult and pediatric patients 12 years of age and older (1.1)
- Reduction in risk of overt hepatic encephalopathy (HE) recurrence in adults (1.2)
- Treatment of irritable bowel syndrome with diarrhea (IBS-D) in adults (1.3)
Limitations of Use
DOSAGE AND ADMINISTRATION
Condition
Recommended Dosage Regimen
TD (2.1)
One 200 mg tablet 3 times a day for 3 days
HE (2.2)
One 550 mg tablet 2 times a day
IBS-D (2.3)
One 550 mg tablet 3 times a day for 14 days. Patients who experience recurrence can be retreated up to two times with the same regimen.
- XIFAXAN can be taken with or without food. (2.4)
DOSAGE FORMS AND STRENGTHS
200 mg and 550 mg tablets (3)
CONTRAINDICATIONS
History of hypersensitivity to rifaximin, rifamycin antimicrobial agents, or any of the components of XIFAXAN (4)
WARNINGS AND PRECAUTIONS
- Travelers’ Diarrhea Not Caused by E. coli: XIFAXAN was not effective in diarrhea complicated by fever and/or blood in the stool or diarrhea due to pathogens other than E. coli. If diarrhea symptoms get worse or persist for more than 24 to 48 hours, discontinue XIFAXAN and consider alternative antibiotics (5.1)
- Clostridium difficile-Associated Diarrhea: Evaluate if diarrhea occurs after therapy or does not improve or worsens during therapy (5.2)
- Hepatic Impairment: Use with caution in patients with severe (Child-Pugh Class C) hepatic impairment (5.4, 8.7)
- Concomitant P-glycoprotein (P-gp) inhibitors (e.g., cyclosporine): Caution should be exercised when concomitant use of XIFAXAN and a P-glycoprotein inhibitor is needed (5.5, 7.1)
ADVERSE REACTIONS
Most common adverse reactions:
- TD (≥2%): Headache (6.1)
- HE (≥10%): Peripheral edema, nausea, dizziness, fatigue, and ascites (6.1)
- IBS-D (≥2%): ALT increased, nausea (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Salix Pharmaceuticals at 1-800-321-4576 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Warfarin: Monitor INR and prothrombin time; dose adjustment of warfarin may be needed to maintain target INR range. (7.2)
USE IN SPECIFIC POPULATIONS
Pregnancy: May cause fetal harm (8.1)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 10/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Travelers’ Diarrhea
1.2 Hepatic Encephalopathy
1.3 Irritable Bowel Syndrome with Diarrhea
2 DOSAGE AND ADMINISTRATION
2.1 Dosage for Travelers’ Diarrhea
2.2 Dosage for Hepatic Encephalopathy
2.3 Dosage for Irritable Bowel Syndrome with Diarrhea
2.4 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Travelers’ Diarrhea Not Caused by Escherichia coli
5.2 Clostridium difficile-Associated Diarrhea
5.3 Development of Drug-Resistant Bacteria
5.4 Severe (Child-Pugh Class C) Hepatic Impairment
5.5 Concomitant Use with P-glycoprotein Inhibitors
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 P-glycoprotein Inhibitors
7.2 Warfarin
7.3 CYP3A4 Substrates
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Travelers’ Diarrhea
14.2 Hepatic Encephalopathy
14.3 Irritable Bowel Syndrome with Diarrhea
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
To reduce the development of drug-resistant bacteria and maintain the effectiveness of XIFAXAN and other antibacterial drugs, XIFAXAN when used to treat infection should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
1.1 Travelers’ Diarrhea
XIFAXAN is indicated for the treatment of travelers’ diarrhea (TD) caused by noninvasive strains of Escherichia coli in adults and pediatric patients 12 years of age and older.
Limitations of Use
XIFAXAN should not be used in patients with diarrhea complicated by fever or blood in the stool or diarrhea due to pathogens other than Escherichia coli [see Warnings and Precautions (5.1), Microbiology (12.4), Clinical Studies (14.1)].
1.2 Hepatic Encephalopathy
XIFAXAN is indicated for reduction in risk of overt hepatic encephalopathy (HE) recurrence in adults.
In the trials of XIFAXAN for HE, 91% of the patients were using lactulose concomitantly. Differences in the treatment effect of those patients not using lactulose concomitantly could not be assessed.
XIFAXAN has not been studied in patients with MELD (Model for End-Stage Liver Disease) scores >25, and only 8.6% of patients in the controlled trial had MELD scores over 19. There is increased systemic exposure in patients with more severe hepatic dysfunction [see Warnings and Precautions (5.4), Use in Specific Populations (8.7), Clinical Pharmacology (12.3)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosage for Travelers’ Diarrhea
The recommended dose of XIFAXAN is one 200 mg tablet taken orally three times a day for 3 days.
2.2 Dosage for Hepatic Encephalopathy
The recommended dose of XIFAXAN is one 550 mg tablet taken orally two times a day.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
XIFAXAN is contraindicated in patients with a hypersensitivity to rifaximin, any of the rifamycin antimicrobial agents, or any of the components in XIFAXAN. Hypersensitivity reactions have included exfoliative dermatitis, angioneurotic edema, and anaphylaxis [see Adverse Reactions (6.2)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Travelers’ Diarrhea Not Caused by Escherichia coli
XIFAXAN was not found to be effective in patients with diarrhea complicated by fever and/or blood in the stool or diarrhea due to pathogens other than Escherichia coli.
Discontinue XIFAXAN if diarrhea symptoms get worse or persist more than 24 to 48 hours and alternative antibiotic therapy should be considered.
XIFAXAN is not effective in cases of travelers’ diarrhea due to Campylobacter jejuni. The effectiveness of XIFAXAN in travelers’ diarrhea caused by Shigella spp. and Salmonella spp. has not been proven. XIFAXAN should not be used in patients where Campylobacter jejuni, Shigella spp., or Salmonella spp. may be suspected as causative pathogens [see Indications and Usage (1.1)].
5.2 Clostridium difficile-Associated Diarrhea
Clostridium difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including XIFAXAN, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon which may lead to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
5.3 Development of Drug-Resistant Bacteria
Prescribing XIFAXAN for travelers’ diarrhea in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
5.4 Severe (Child-Pugh Class C) Hepatic Impairment
There is increased systemic exposure in patients with severe hepatic impairment. The clinical trials were limited to patients with MELD scores <25. Therefore, caution should be exercised when administering XIFAXAN to patients with severe hepatic impairment (Child-Pugh Class C) [see Use in Specific Populations (8.7), Clinical Studies (14.2)].
5.5 Concomitant Use with P-glycoprotein Inhibitors
Concomitant administration of drugs that are P-glycoprotein (P-gp) inhibitors with XIFAXAN can substantially increase the systemic exposure to rifaximin. Caution should be exercised when concomitant use of XIFAXAN and a P-gp inhibitor such as cyclosporine is needed. In patients with hepatic impairment, a potential additive effect of reduced metabolism and concomitant P-gp inhibitors may further increase the systemic exposure to rifaximin [see Drug Interactions (7.1), Clinical Pharmacology (12.3)].
-
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Travelers’ Diarrhea
The safety of XIFAXAN 200 mg taken three times a day was evaluated in patients with travelers’ diarrhea consisting of 320 patients in two placebo-controlled clinical trials with 95% of patients receiving three or four days of treatment with XIFAXAN. The population studied had a mean age of 31.3 (18-79) years of which approximately 3% were ≥65 years old, 53% were male and 84% were White, 11% were Hispanic.
Discontinuations due to adverse reactions occurred in 0.4% of patients. The adverse reactions leading to discontinuation were taste loss, dysentery, weight decrease, anorexia, nausea and nasal passage irritation.
The adverse reaction that occurred at a frequency ≥2% in XIFAXAN-treated patients (n=320) at a higher rate than placebo (n=228) in the two placebo-controlled trials of TD was:
- headache (10% XIFAXAN, 9% placebo)
Hepatic Encephalopathy
The data described below reflect exposure to XIFAXAN in 348 patients, including 265 exposed for 6 months and 202 exposed for more than a year (mean exposure was 364 days). The safety of XIFAXAN 550 mg taken two times a day for reducing the risk of overt hepatic encephalopathy recurrence in adult patients was evaluated in a 6-month placebo-controlled clinical trial (n=140) and in a long term follow-up study (n=280). The population studied had a mean age of 56 (range: 21 to 82) years; approximately 20% of the patients were ≥65 years old, 61% were male, 86% were White, and 4% were Black. Ninety-one percent of patients in the trial were taking lactulose concomitantly. The most common adverse reactions that occurred at an incidence ≥5% and at a higher incidence in XIFAXAN-treated subjects than in the placebo group in the 6-month trial are provided in Table 1.
Table 1: Most Common Adverse Reactions in HE Trial Number (%) of Patients
MedDRA Preferred Term
XIFAXAN Tablets
550 mg TWICE DAILY
n=140
Placebo
n=159
Peripheral edema
21 (15%)
13 (8%)
Nausea
20 (14%)
21 (13%)
Dizziness
18 (13%)
13 (8%)
Fatigue
17 (12%)
18 (11%)
Ascites
16 (11%)
15 (9%)
Muscle spasms
13 (9%)
11 (7%)
Pruritus
13 (9%)
10 (6%)
Abdominal pain
12 (9%)
13 (8%)
Anemia
11 (8%)
6 (4%)
Depression
10 (7%)
8 (5%)
Nasopharyngitis
10 (7%)
10 (6%)
Abdominal pain upper
9 (6%)
8 (5%)
Arthralgia
9 (6%)
4 (3%)
Dyspnea
9 (6%)
7 (4%)
Pyrexia
9 (6%)
5 (3%)
Rash
7 (5%)
- 6 (4%)
Irritable Bowel Syndrome with Diarrhea
The safety of XIFAXAN for the treatment of IBS-D was evaluated in 3 placebo-controlled studies in which 952 patients were randomized to XIFAXAN 550 mg three times a day for 14 days. Across the 3 studies, 96% of patients received at least 14 days of treatment with XIFAXAN. In Trials 1 and 2, 624 patients received only one 14-day treatment. Trial 3 evaluated the safety of XIFAXAN in 328 patients who received 1 open-label treatment and 2 double-blind repeat treatments of 14 days each over a period of up to 46 weeks. The combined population studied had a mean age of 47 (range: 18 to 88) years of whom approximately 11% of the patients were ≥65 years old, 72% were female, 88% were White, 9% were Black and 12% were Hispanic.
The adverse reaction that occurred at a frequency ≥2% in XIFAXAN-treated patients at a higher rate than placebo in Trials 1 and 2 for IBS-D was:
- nausea (3% XIFAXAN, 2% placebo)
The adverse reactions that occurred at a frequency ≥2% in XIFAXAN-treated patients (n=328) at a higher rate than placebo (n=308) in Trial 3 for IBS-D during the double-blind treatment phase were:
- ALT increased (XIFAXAN 2%, placebo 1%)
- nausea (XIFAXAN 2%, placebo 1%)
Less Common Adverse Reactions
The following adverse reactions, presented by body system, were reported in less than 2% of patients in clinical trials of TD and IBS-D and in less than 5% of patients in clinical trials of HE:
Hepatobiliary disorders: Clostridium colitis
Investigations: Increased blood creatine phosphokinase
Musculoskeletal and connective tissue disorders: myalgia
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of XIFAXAN. Because these reactions are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These reactions have been chosen for inclusion due to either their seriousness, frequency of reporting or causal connection to XIFAXAN.
Infections and Infestations
Cases of C. difficile-associated colitis have been reported [see Warnings and Precautions (5.2)].
General
Hypersensitivity reactions, including exfoliative dermatitis, rash, angioneurotic edema (swelling of face and tongue and difficulty swallowing), urticaria, flushing, pruritus and anaphylaxis have been reported. These events occurred as early as within 15 minutes of drug administration.
-
7 DRUG INTERACTIONS
7.1 P-glycoprotein Inhibitors
Concomitant administration of cyclosporine, an inhibitor of P-gp and OATPs significantly increased the systemic exposure of rifaximin. In patients with hepatic impairment, a potential additive effect of reduced metabolism and concomitant P-gp inhibitors may further increase the systemic exposure to rifaximin. Caution should be exercised when concomitant use of XIFAXAN and a P-gp inhibitor such as cyclosporine is needed [see Warnings and Precautions (5.5), Clinical Pharmacology (12.3)].
7.2 Warfarin
Changes in INR have been reported postmarketing in patients receiving rifaximin and warfarin concomitantly. Monitor INR and prothrombin time. Dose adjustment of warfarin may be needed to maintain target INR range. See prescribing information for warfarin.
7.3 CYP3A4 Substrates
An in vitro study has suggested that rifaximin induces CYP3A4 [see Clinical Pharmacology (12.3)]. However, in patients with normal liver function, XIFAXAN at the recommended dosing regimen is not expected to induce CYP3A4. It is unknown whether rifaximin can have a significant effect on the pharmacokinetics of concomitant CYP3A4 substrates in patients with reduced liver function who have elevated rifaximin concentrations.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on XIFAXAN use in pregnant women to inform any drug associated risks. Teratogenic effects were observed in animal reproduction studies following administration of rifaximin to pregnant rats and rabbits during organogenesis at doses approximately 0.9 to 5 times and 0.7 to 33 times, respectively of the recommended human doses of 600 mg to 1650 mg per day. In rabbits, ocular, oral and maxillofacial, cardiac, and lumbar spine malformations were observed. Ocular malformations were observed in both rats and rabbits at doses that caused reduced maternal body weight gain [see Data]. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively. Advise pregnant women of the potential risk to a fetus.
Data
Animal Data
Rifaximin was teratogenic in rats at doses of 150 to 300 mg/kg (approximately 2.5 to 5 times the recommended dose for TD [600 mg per day], and approximately 1.3 to 2.6 times the recommended dose for HE [1100 mg per day], and approximately 0.9 to 1.8 times the recommended dose for IBS-D [1650 mg per day] adjusted for body surface area). Rifaximin was teratogenic in rabbits at doses of 62.5 to 1000 mg/kg (approximately 2 to 33 times the recommended dose for TD [600 mg per day], and approximately 1.1 to 18 times the recommended dose for HE [1100 mg per day], and approximately 0.7 to 12 times the recommended dose for IBS-D [1650 mg per day] adjusted for body surface area). These effects include cleft palate, agnathia, jaw shortening, hemorrhage, eye partially open, small eyes, brachygnathia, incomplete ossification, and increased thoracolumbar vertebrae.
A pre and postnatal development study in rats showed no evidence of any adverse effect on pre and postnatal development at oral doses of rifaximin up to 300 mg/kg per day (approximately 5 times the recommended dose for TD [600 mg per day], and approximately 2.6 times the recommended dose for HE [1100 mg per day], and approximately 1.8 times the recommended dose for IBS-D [1650 mg per day] adjusted for body surface area).
8.2 Lactation
Risk Summary
There is no information regarding the presence of rifaximin in human milk, the effects of rifaximin on the breastfed infant, or the effects of rifaximin on milk production. The development and health benefits of breastfeeding should be considered along with the mother’s clinical need for XIFAXAN and any potential adverse effects on the breastfed infant from XIFAXAN or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of XIFAXAN has not been established in pediatric patients less than 12 years of age with TD or in patients less than 18 years of age for HE and IBS-D.
8.5 Geriatric Use
Of the total number of patients in the clinical study of XIFAXAN for HE, 19% of patients were 65 and over, while 2% were 75 and over. In the clinical studies of IBS-D, 11% of patients were 65 and over, while 2% were 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects for either indication. Clinical studies with XIFAXAN for TD did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently than younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Renal Impairment
The pharmacokinetics of rifaximin in patients with impaired renal function has not been studied.
8.7 Hepatic Impairment
Following administration of XIFAXAN 550 mg twice daily to patients with a history of hepatic encephalopathy, the systemic exposure (i.e., AUCτ) of rifaximin was about 10-, 14-, and 21-fold higher in those patients with mild (Child-Pugh Class A), moderate (Child-Pugh Class B) and severe (Child-Pugh Class C) hepatic impairment, respectively, compared to that in healthy volunteers. No dosage adjustment is recommended because rifaximin is presumably acting locally. Nonetheless, caution should be exercised when XIFAXAN is administered to patients with severe hepatic impairment [see Warnings and Precautions (5.4), Clinical Pharmacology (12.3), Clinical Studies (14.2)].
-
10 OVERDOSAGE
No specific information is available on the treatment of overdosage with XIFAXAN. In clinical studies at doses higher than the recommended dose (greater than 600 mg per day for TD, greater than 1100 mg per day for HE or greater than 1650 mg per day for IBS-D), adverse reactions were similar in subjects who received doses higher than the recommended dose and placebo. In the case of overdosage, discontinue XIFAXAN, treat symptomatically, and institute supportive measures as required.
-
11 DESCRIPTION
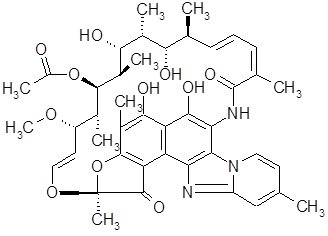
XIFAXAN tablets contain rifaximin, a non-aminoglycoside semi-synthetic, nonsystemic antibiotic derived from rifamycin SV. Rifaximin is a structural analog of rifampin. The chemical name for rifaximin is (2S,16Z,18E,20S,21S,22R,23R,24R,25S,26S,27S,28E)-5,6,21,23,25-pentahydroxy-27-methoxy-2,4,11,16,20,22,24,26-octamethyl-2,7-(epoxypentadeca-[1,11,13]trienimino)benzofuro[4,5-e]pyrido[1,2-á]-benzimidazole-1,15(2H)-dione,25-acetate. The empirical formula is C43H51N3O11 and its molecular weight is 785.9. The chemical structure is represented below:
XIFAXAN tablets for oral administration are film-coated and contain 200 mg or 550 mg of rifaximin.
Inactive ingredients:
Each 200 mg tablet contains colloidal silicon dioxide, disodium edetate, glycerol palmitostearate, hypromellose, microcrystalline cellulose, propylene glycol, red iron oxide, sodium starch glycolate, talc, and titanium dioxide.
Each 550 mg tablet contains colloidal silicon dioxide, glycerol palmitostearate, microcrystalline cellulose, polyethylene glycol/macrogol, polyvinyl alcohol, red iron oxide, sodium starch glycolate, talc, and titanium dioxide.
-
12 CLINICAL PHARMACOLOGY
12.3 Pharmacokinetics
Absorption
In healthy subjects, the mean time to reach peak rifaximin plasma concentrations was about an hour and the mean Cmax ranged 2.4 to 4 ng/mL after a single dose and multiple doses of XIFAXAN 550 mg.
Travelers’ Diarrhea
Systemic absorption of XIFAXAN (200 mg three times daily) was evaluated in 13 subjects challenged with shigellosis on Days 1 and 3 of a three-day course of treatment. Rifaximin plasma concentrations and exposures were low and variable. There was no evidence of accumulation of rifaximin following repeated administration for 3 days (9 doses). Peak plasma rifaximin concentrations after 3 and 9 consecutive doses ranged from 0.81 to 3.4 ng/mL on Day 1 and 0.68 to 2.26 ng/mL on Day 3. Similarly, AUC0-last estimates were 6.95 ± 5.15 ngh/mL on Day 1 and 7.83 ± 4.94 ngh/mL on Day 3. XIFAXAN is not suitable for treating systemic bacterial infections because of limited systemic exposure after oral administration [see Warnings and Precautions (5.1)].
Hepatic Encephalopathy
Mean rifaximin exposure (AUCτ) in patients with a history of HE was approximately 12-fold higher than that observed in healthy subjects. Among patients with a history of HE, the mean AUC in patients with Child-Pugh Class C hepatic impairment was 2-fold higher than in patients with Child-Pugh Class A hepatic impairment [see Warnings and Precautions (5.4) and Use in Specific Populations (8.7)].
Irritable Bowel Syndrome with Diarrhea
In patients with irritable bowel syndrome with diarrhea (IBS-D) treated with XIFAXAN 550 mg three times a day for 14 days, the median Tmax was 1 hour and mean Cmax and AUC were generally comparable with those in healthy subjects. After multiple doses, AUCtau was 1.65-fold higher than that on Day 1 in IBS-D patients (Table 2).
Table 2. Mean (± SD) Pharmacokinetic Parameters of Rifaximin Following XIFAXAN 550 mg Three Times a Day in IBS-D Patients and Healthy Subjects Healthy Subjects
IBS-D Patients
Single-
Dose
(Day 1)
n=12Multiple-
Dose
(Day 14)
n=14Single-
Dose
(Day 1)
n=24Multiple-
Dose
(Day 14)
n=24Cmax
(ng/mL)4.04
(1.51)2.39
(1.28)3.49
(1.36)4.22
(2.66)Tmax
(h)0.75
(0.5-2.1)1.00
(0.5-2.0)0.78
(0-2)1.00
(0.5-2)AUCtau
(ngh/mL)10.4
(3.47)9.30
(2.7)9.69
(4.16)16.0
(9.59)Half-life
(h)1.83
(1.38)5.63
(5.27)3.14
(1.71)6.08
(1.68)Food Effect in Healthy Subjects
A high-fat meal consumed 30 minutes prior to XIFAXAN dosing in healthy subjects delayed the mean time to peak plasma concentration from 0.75 to 1.5 hours and increased the systemic exposure (AUC) of rifaximin by 2-fold but did not significantly affect Cmax.
Distribution
Rifaximin is moderately bound to human plasma proteins. In vivo, the mean protein binding ratio was 67.5% in healthy subjects and 62% in patients with hepatic impairment when XIFAXAN was administered.
Elimination
The mean half-life of rifaximin in healthy subjects at steady-state was 5.6 hours and was 6 hours in IBS-D patients.
Metabolism
In an in vitro study rifaximin was metabolized mainly by CYP3A4. Rifaximin accounted for 18% of radioactivity in plasma suggesting that the absorbed rifaximin undergoes extensive metabolism.
Excretion
In a mass balance study, after administration of 400 mg 14C-rifaximin orally to healthy volunteers, of the 96.94% total recovery, 96.62% of the administered radioactivity was recovered in feces mostly as the unchanged drug and 0.32% was recovered in urine mostly as metabolites with 0.03% as the unchanged drug.
Biliary excretion of rifaximin was suggested by a separate study in which rifaximin was detected in the bile after cholecystectomy in patients with intact gastrointestinal mucosa.
Specific Populations
Hepatic Impairment
The systemic exposure of rifaximin was markedly elevated in patients with hepatic impairment compared to healthy subjects.
The pharmacokinetics of rifaximin in patients with a history of HE was evaluated after administration of XIFAXAN 550 mg twice a day. The pharmacokinetic parameters were associated with a high variability and mean rifaximin exposure (AUCτ) in patients with a history of HE was higher compared to those in healthy subjects. The mean AUCτ in patients with hepatic impairment of Child-Pugh Class A, B, and C was 10-, 14-, and 21-fold higher, respectively, compared to that in healthy subjects (Table 3).
Table 3. Mean (± SD) Pharmacokinetic Parameters of Rifaximin at Steady-State in Patients with a History of Hepatic Encephalopathy by Child-Pugh Class* - * Cross-study comparison with pharmacokinetic parameters in healthy subjects
- † Median (range)
Healthy
Subjects
(n=14)Child-Pugh Class
A (n=18)
B (n=15)
C (n=6)
AUCtau
(ngh/mL)12.3 ± 4.8
118 ± 67.8
169 ± 55.7
257 ± 100.2
Cmax
(ng/mL)3.4 ± 1.6
19.5 ± 11.4
25.4 ± 11.9
39.7 ± 13.4
Tmax†
(h)0.8 (0.5, 4.0)
1 (0.9, 10)
1 (1.0, 4.2)
- 1 (0, 2)
Renal Impairment
The pharmacokinetics of rifaximin in patients with impaired renal function has not been studied.
Drug Interaction Studies
Effect of Other Drugs on Rifaximin
An in vitro study suggests that rifaximin is a substrate of CYP3A4.
In vitro rifaximin is a substrate of P-glycoprotein, OATP1A2, OATP1B1, and OATP1B3. Rifaximin is not a substrate of OATP2B1.
Cyclosporine
In vitro in the presence of P-glycoprotein inhibitor, verapamil, the efflux ratio of rifaximin was reduced greater than 50%. In a clinical drug interaction study, mean Cmax for rifaximin was increased 83-fold, from 0.48 to 40.0 ng/mL; mean AUC∞ was increased 124-fold, from 2.54 to 314 ng●h/mL following co-administration of a single dose of XIFAXAN 550 mg with a single 600 mg dose of cyclosporine, an inhibitor of P-glycoprotein [see Drug Interactions (7.1)].
Cyclosporine is also an inhibitor of OATP, breast cancer resistance protein (BCRP) and a weak inhibitor of CYP3A4. The relative contribution of inhibition of each transporter by cyclosporine to the increase in rifaximin exposure is unknown.
Effect of Rifaximin on Other Drugs
In in vitro drug interaction studies the IC50 values for rifaximin was >50 micromolar (~60 mcg) for CYP isoforms 1A2, 2A6, 2B6, 2C9, 2C19, 2D6, and 2E1. In vitro IC50 value of rifaximin for CYP3A4 was 25 micromolar. Based on in vitro studies, clinically significant drug interaction via inhibition of 1A2, 2A6, 2B6, 2C9, 2C19, 2D6, 2E1 and 3A4 by rifaximin is not expected.
The inhibitory effect of rifaximin on P-glycoprotein transport was observed in an in vitro study. The effect of rifaximin on P-gp transporter was not evaluated in vivo.
In in vitro studies, rifaximin at 3 micromolar inhibited the uptake of estradiol glucuronide via OATP1B1 by 64% and via OATP1B3 by 70% while the uptake of estrone sulfate via OATP1A2 was inhibited by 40%. The inhibitory potential of rifaximin on these transporters at the clinically relevant concentrations is unknown.
Midazolam
In an in vitro study, rifaximin was shown to induce CYP3A4 at the concentration of 0.2 micromolar. No significant induction of CYP3A4 enzyme using midazolam as a substrate was observed when rifaximin was administered three times a day for 7 days at 200 mg and 550 mg doses in two clinical drug interaction studies in healthy subjects.
The effect of XIFAXAN 200 mg administered orally every 8 hours for 3 days and for 7 days on the pharmacokinetics of a single dose of either 2 mg intravenous midazolam or 6 mg oral midazolam was evaluated in healthy subjects. No significant difference was observed in the systemic exposure or elimination of intravenous or oral midazolam or its major metabolite, 1’-hydroxymidazolam, between midazolam alone or together with XIFAXAN. Therefore, XIFAXAN was not shown to significantly affect intestinal or hepatic CYP3A4 activity for the 200 mg three times a day dosing regimen.
When single dose of 2 mg midazolam was orally administered after administration of XIFAXAN 550 mg three times a day for 7 days and 14 days to healthy subjects, the mean AUC of midazolam was 3.8% and 8.8% lower, respectively, than when midazolam was administered alone. The mean Cmax of midazolam was lower by 4 to 5% when XIFAXAN was administered for 7-14 days prior to midazolam administration. This degree of interaction is not considered clinically meaningful.
Oral Contraceptives Containing Ethinyl Estradiol and Norgestimate
The oral contraceptive study utilized an open-label, crossover design in 28 healthy female subjects to determine if XIFAXAN 200 mg orally administered three times a day for 3 days (the dosing regimen for travelers’ diarrhea) altered the pharmacokinetics of a single dose of an oral contraceptive containing 0.07 mg ethinyl estradiol and 0.5 mg norgestimate. Results showed that the pharmacokinetics of single doses of ethinyl estradiol and norgestimate were not altered by XIFAXAN.
An open-label oral contraceptive study was conducted in 39 healthy female subjects to determine if XIFAXAN 550 mg orally administered three times a day for 7 days altered the pharmacokinetics of a single dose of an oral contraceptive containing 0.025 mg of ethinyl estradiol (EE) and 0.25 mg norgestimate (NGM). Mean Cmax of EE and NGM was lower by 25% and 13%, after the 7-day XIFAXAN regimen than when the oral contraceptive was given alone. The mean AUC values of NGM active metabolites were lower by 7% to approximately 11%, while AUC of EE was not altered in presence of rifaximin. The clinical relevance of the Cmax and AUC reductions in the presence of rifaximin is not known.
12.4 Microbiology
Mechanism of Action
Rifaximin is a semi-synthetic derivative of rifampin and acts by binding to the beta-subunit of bacterial DNA-dependent RNA polymerase blocking one of the steps in transcription. This results in inhibition of bacterial protein synthesis and consequently inhibits the growth of bacteria.
Drug Resistance and Cross-Resistance
Resistance to rifaximin is caused primarily by mutations in the rpoB gene. This changes the binding site on DNA dependent RNA polymerase and decreases rifaximin binding affinity, thereby reducing efficacy. Cross-resistance between rifaximin and other classes of antimicrobials has not been observed.
Antibacterial Activity
Rifaximin has been shown to be active against the following pathogens both in vitro and in clinical studies of infectious diarrhea as described in the Indications and Usage (1.1) section:
Escherichia coli (enterotoxigenic and enteroaggregative strains).
Susceptibility Tests
In vitro susceptibility testing was performed according to the Clinical and Laboratory Standards Institute (CLSI).1,2,3 However, the correlation between susceptibility testing and clinical outcome has not been determined.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Malignant schwannomas in the heart were significantly increased in male Crl:CD (SD) rats that received rifaximin by oral gavage for two years at 150 to 250 mg/kg per day (doses equivalent to 2.4 to 4 times the recommended dose of 200 mg three times daily for TD, and equivalent to 1.3 to 2.2 times the recommended dose of 550 mg twice daily for HE, based on relative body surface area comparisons). There was no increase in tumors in Tg.rasH2 mice dosed orally with rifaximin for 26 weeks at 150 to 2000 mg/kg per day (doses equivalent to 1.2 to 16 times the recommended daily dose for TD and equivalent to 0.7 to 9 times the recommended daily dose for HE, based on relative body surface area comparisons).
Rifaximin was not genotoxic in the bacterial reverse mutation assay, chromosomal aberration assay, rat bone marrow micronucleus assay, rat hepatocyte unscheduled DNA synthesis assay, or the CHO/HGPRT mutation assay. There was no effect on fertility in male or female rats following the administration of rifaximin at doses up to 300 mg/kg (approximately 5 times the clinical dose of 600 mg per day for TD, and approximately 2.6 times the clinical dose of 1100 mg per day for HE, adjusted for body surface area).
-
14 CLINICAL STUDIES
14.1 Travelers’ Diarrhea
The efficacy of XIFAXAN given as 200 mg orally taken three times a day for 3 days was evaluated in 2 randomized, multi‑center, double-blind, placebo-controlled studies in adult subjects with travelers’ diarrhea. One study was conducted at clinical sites in Mexico, Guatemala, and Kenya (Study 1). The other study was conducted in Mexico, Guatemala, Peru, and India (Study 2). Stool specimens were collected before treatment and 1 to 3 days following the end of treatment to identify enteric pathogens. The predominant pathogen in both studies was Escherichia coli.
The clinical efficacy of XIFAXAN was assessed by the time to return to normal, formed stools and resolution of symptoms. The primary efficacy endpoint was time to last unformed stool (TLUS) which was defined as the time to the last unformed stool passed, after which clinical cure was declared. Table 4 displays the median TLUS and the number of patients who achieved clinical cure for the intent to treat (ITT) population of Study 1. The duration of diarrhea was significantly shorter in patients treated with XIFAXAN than in the placebo group. More patients treated with XIFAXAN were classified as clinical cures than were those in the placebo group.
Table 4. Clinical Response in Study 1 (ITT population) XIFAXAN
(n=125)Placebo
(n=129)Estimate
(97.5% CI)Median TLUS
(hours)32.5
58.6
2
(1.26, 2.50)Clinical cure,
n (%)99
(79)78
(60)19
(5.3, 32.1)
Microbiological eradication (defined as the absence of a baseline pathogen in culture of stool after 72 hours of therapy) rates for Study 1 are presented in Table 5 for patients with any pathogen at baseline and for the subset of patients with Escherichia coli at baseline. Escherichia coli was the only pathogen with sufficient numbers to allow comparisons between treatment groups.Even though XIFAXAN had microbiologic activity similar to placebo, it demonstrated a clinically significant reduction in duration of diarrhea and a higher clinical cure rate than placebo. Therefore, patients should be managed based on clinical response to therapy rather than microbiologic response.
Table 5. Microbiologic Eradication Rates in Study 1 Subjects with a Baseline Pathogen XIFAXAN
Placebo
Overall
48/70 (69)
41/61 (67)
E. coli
38/53 (72)
40/54 (74)
The results of Study 2 supported the results presented for Study 1. In addition, this study provided evidence that subjects treated with XIFAXAN with fever and/or blood in the stool at baseline had prolonged TLUS. These subjects had lower clinical cure rates than those without fever or blood in the stool at baseline. Many of the patients with fever and/or blood in the stool (dysentery-like diarrheal syndromes) had invasive pathogens, primarily Campylobacter jejuni, isolated in the baseline stool.
Also in this study, the majority of the subjects treated with XIFAXAN who had Campylobacter jejuni isolated as a sole pathogen at baseline failed treatment and the resulting clinical cure rate for these patients was 23.5% (4/17). In addition to not being different from placebo, the microbiologic eradication rates for subjects with Campylobacter jejuni isolated at baseline were much lower than the eradication rates seen for Escherichia coli.
In an unrelated open-label, pharmacokinetic study of oral XIFAXAN 200 mg taken every 8 hours for 3 days, 15 adult subjects were challenged with Shigella flexneri 2a, of whom 13 developed diarrhea or dysentery and were treated with XIFAXAN. Although this open-label challenge trial was not adequate to assess the effectiveness of XIFAXAN in the treatment of shigellosis, the following observations were noted: eight subjects received rescue treatment with ciprofloxacin either because of lack of response to XIFAXAN treatment within 24 hours (2), or because they developed severe dysentery (5), or because of recurrence of Shigella flexneri in the stool (1); five of the 13 subjects received ciprofloxacin although they did not have evidence of severe disease or relapse.
14.2 Hepatic Encephalopathy
The efficacy of XIFAXAN 550 mg taken orally two times a day was evaluated in a randomized, placebo-controlled, double-blind, multi-center 6-month trial of adult subjects from the U.S., Canada and Russia who were defined as being in remission (Conn score of 0 or 1) from hepatic encephalopathy (HE). Eligible subjects had ≥2 episodes of HE associated with chronic liver disease in the previous 6 months.
A total of 299 subjects were randomized to receive either XIFAXAN (n=140) or placebo (n=159) in this study. Patients had a mean age of 56 years (range, 21-82 years), 81% <65 years of age, 61% were male and 86% White. At baseline, 67% of patients had a Conn score of 0 and 68% had an asterixis grade of 0. Patients had MELD scores of either ≤10 (27%) or 11 to 18 (64%) at baseline. No patients were enrolled with a MELD score of >25. Nine percent of the patients were Child-Pugh Class C. Lactulose was concomitantly used by 91% of the patients in each treatment arm of the study. Per the study protocol, patients were withdrawn from the study after experiencing a breakthrough HE episode. Other reasons for early study discontinuation included: adverse reactions (XIFAXAN 6%; placebo 4%), patient request to withdraw (XIFAXAN 4%; placebo 6%) and other (XIFAXAN 7%; placebo 5%).
The primary endpoint was the time to first breakthrough overt HE episode. A breakthrough overt HE episode was defined as a marked deterioration in neurological function and an increase of Conn score to Grade ≥2. In patients with a baseline Conn score of 0, a breakthrough overt HE episode was defined as an increase in Conn score of 1 and asterixis grade of 1.
Breakthrough overt HE episodes were experienced by 31 of 140 subjects (22%) in the XIFAXAN group and by 73 of 159 subjects (46%) in the placebo group during the 6-month treatment period. Comparison of Kaplan-Meier estimates of event-free curves showed XIFAXAN significantly reduced the risk of HE breakthrough by 58% during the 6-month treatment period. Presented below in Figure 1 is the Kaplan-Meier event-free curve for all subjects (n=299) in the study.
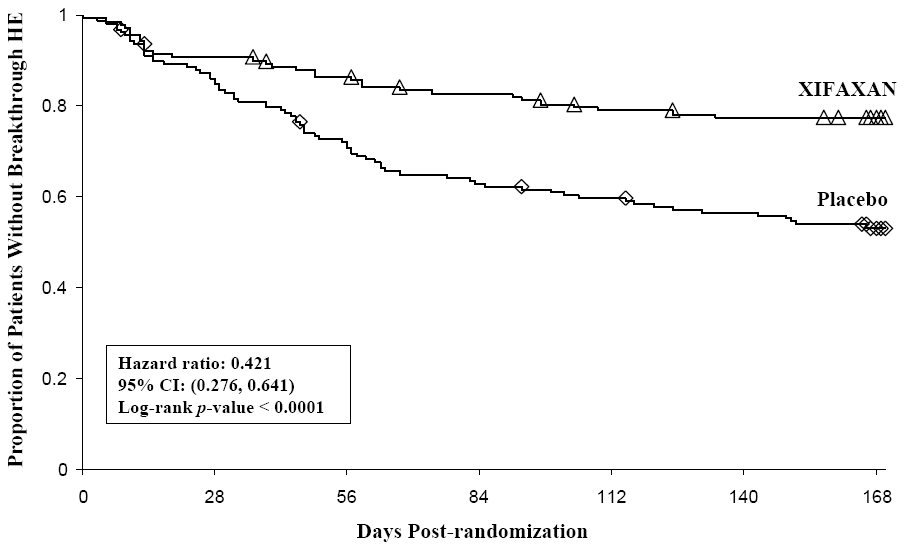
Figure 1: Kaplan-Meier Event-Free Curves1 in HE Study (Time to First Breakthrough-HE Episode up to 6 Months of Treatment, Day 170) (ITT Population)
Note: Open diamonds and open triangles represent censored subjects.
1 Event-free refers to non-occurrence of breakthrough HE.When the results were evaluated by the following demographic and baseline characteristics, the treatment effect of XIFAXAN 550 mg in reducing the risk of breakthrough overt HE recurrence was consistent for: sex, baseline Conn score, duration of current remission and diabetes. The differences in treatment effect could not be assessed in the following subpopulations due to small sample size: non-White (n=42), baseline MELD >19 (n=26), Child-Pugh Class C (n=31), and those without concomitant lactulose use (n=26).
HE-related hospitalizations (hospitalizations directly resulting from HE, or hospitalizations complicated by HE) were reported for 19 of 140 subjects (14%) and 36 of 159 subjects (23%) in the XIFAXAN (rifaximin) and placebo groups respectively. Comparison of Kaplan-Meier estimates of event-free curves showed XIFAXAN significantly reduced the risk of HE-related hospitalizations by 50% during the 6-month treatment period. Comparison of Kaplan-Meier estimates of event-free curves is shown in Figure 2.
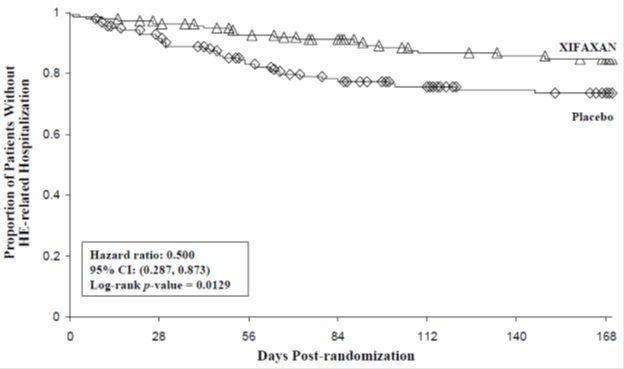
Figure 2: Kaplan-Meier Event-Free Curves1 in Pivotal HE Study (Time to First HE-Related Hospitalization in HE Study up to 6 Months of Treatment, Day 170) (ITT Population)
Note: Open diamonds and open triangles represent censored subjects.
1 Event-free refers to non-occurrence of HE-related hospitalization.14.3 Irritable Bowel Syndrome with Diarrhea
The efficacy of XIFAXAN for the treatment of IBS-D was established in 3 randomized, multi‑center, double-blind, placebo-controlled trials in adult patients.
Trials 1 and 2 - Design
The first two trials, Trials 1 and 2 were of identical design. In these trials, a total of 1258 patients meeting Rome II criteria for IBS* were randomized to receive XIFAXAN 550 mg three times a day (n=624) or placebo (n=634) for 14 days and then followed for a 10-week treatment-free period. The Rome II criteria further categorizes IBS patients into 3 subtypes: diarrhea-predominant IBS (IBS-D), constipation-predominant IBS (IBS-C), or alternating IBS (bowel habits alternating between diarrhea and constipation). Patients with both IBS-D and alternating IBS were included in Trials 1 and 2. XIFAXAN is recommended for use in patients with IBS-D.
*Rome II Criteria: At least 12 weeks, which need not be consecutive, in the preceding 12 months of abdominal discomfort or pain that has two out of three features: 1. Relieved with defecation; and/or 2. Onset associated with a change in frequency of stool; and/or 3. Onset associated with a change in form (appearance) of stool.
Symptoms that Cumulatively Support the Diagnosis of Irritable Bowel Syndrome:
– Abnormal stool frequency (for research purposes “abnormal” may be defined as greater than 3 bowel movements per day and less than 3 bowel movements per week); Abnormal stool form (lumpy/hard or loose/watery stool); Abnormal stool passage (straining, urgency, or feeling of incomplete evacuation); Passage of mucus; Bloating or feeling of abdominal distension.
Trial 3 - Design
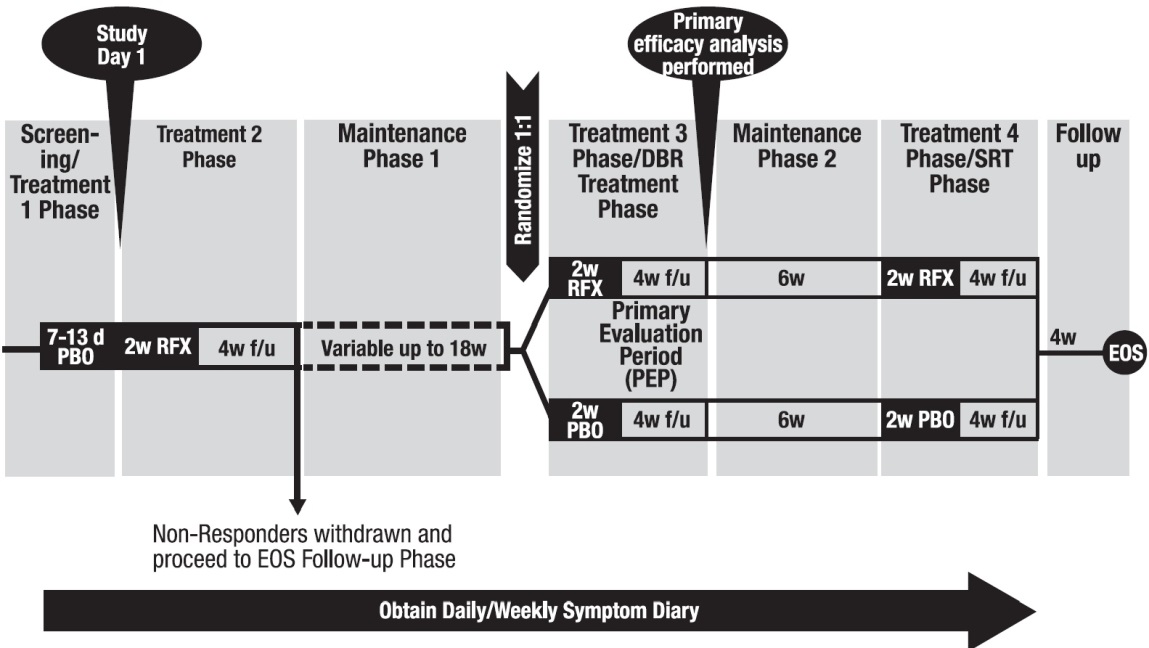
Trial 3 evaluated repeat treatment in adults with IBS-D meeting Rome III criteria** for up to 46 weeks. A total of 2579 were enrolled to receive open-label XIFAXAN for 14 days. Of 2438 evaluable patients, 1074 (44%) responded to initial treatment and were evaluated over 22 weeks for continued response or recurrence of IBS-symptoms. A total of 636 patients had symptom recurrence and were randomized into the double-blind phase of the study. These patients were scheduled to receive XIFAXAN 550 mg three times a day (n=328) or placebo (n=308) for two additional 14-day repeat treatment courses separated by 10 weeks. See Figure 3.
The IBS-D population from the three studies had mean age of 47 (range: 18 to 88) years of which approximately 11% of patients were ≥65 years old, 72% were female and 88% were White.
**Rome III Criteria: Recurrent abdominal pain or discomfort (uncomfortable sensation not described as pain) at least 3 days/month in last 3 months associated with two or more of the following: 1. Improvement with defecation; 2. Onset associated with a change in frequency of stool; 3. Onset associated with a change in form (appearance) of stool.
Trials 1 and 2 - Results
Trials 1 and 2 included 1258 IBS-D patients (309 XIFAXAN, 314 placebo); (315 XIFAXAN, 320 placebo). The primary endpoint for both trials was the proportion of patients who achieved adequate relief of IBS signs and symptoms for at least 2 of 4 weeks during the month following 14 days of treatment. Adequate relief was defined as a response of “yes” to the following weekly Subject Global Assessment (SGA) question: “In regards to your IBS symptoms, compared to the way you felt before you started study medication, have you, in the past 7 days, had adequate relief of your IBS symptoms? [Yes/No].”
Adequate relief of IBS symptoms was experienced by more patients receiving XIFAXAN than those receiving placebo during the month following 2 weeks of treatment (SGA-IBS Weekly Results: 41% vs. 31%, p=0.0125; 41% vs. 32%, p=0.0263 (See Table 6).
Table 6. Adequate Relief of IBS Symptoms During the Month Following Two Weeks of Treatment - * b The p-value for the primary endpoint for Trial 1 and for Trial 2 was <0.05.
Endpoint
Trial 1
XIFAXAN
n=309
n (%)Placebo
n=314
n (%)Treatment
Difference
(95% CI)Adequate Relief of IBS Symptoms*
126
(41)98
(31)10%
(2.1%, 17.1%)Endpoint
Trial 2
XIFAXAN
n=315
n (%)Placebo
n=320
n (%)Treatment
Difference
(95% CI)Adequate Relief of IBS Symptoms
128
(41)103
(32)8%
(1.0%, 15.9%)The trials examined a composite endpoint which defined responders by IBS-related abdominal pain and stool consistency measures. Patients were monthly responders if they met both of the following criteria:
- experienced a ≥30% decrease from baseline in abdominal pain for ≥2 weeks during the month following 2 weeks of treatment
- had a weekly mean stool consistency score <4 (loose stool) for ≥2 weeks during the month following 2 weeks of treatment
More patients receiving XIFAXAN were monthly responders for abdominal pain and stool consistency in Trials 1 and 2 (see Table 7).
Table 7. Efficacy Responder Rates in Trial 1 and 2 During the Month Following Two Weeks of Treatment Endpoint
Trial 1
XIFAXAN
n=309
n (%)Placebo
n=314
n (%)Treatment
Difference
(95% CI)Abdominal
Pain and Stool
Consistency
Responders144
(47)121
(39)8%
(0.3%, 15.9%)Abdominal Pain
Responders159
(51)132
(42)9%
(1.8%, 17.5%)Stool Consistency
Responders244
(79)212
(68)11%
(4.4%, 18.2%)Endpoint
Trial 2
XIFAXAN
n=315
n (%)Placebo
n=320
n (%)Treatment
Difference
(95% CI)Abdominal
Pain and Stool
Consistency
Responders147
(47)116
(36)11% (2.7%,
18.0%)Abdominal Pain
Responders165
(52)138
(43)9%
(1.5%, 17.0%)Stool Consistency
Responders233
(74)206
(64)10%
(2.3%, 16.7%)Trial 3 - Results
In TARGET 3, 2579 patients were scheduled to receive an initial 14-day course of open-label XIFAXAN followed by 4 weeks of treatment-free follow-up. At the end of the follow-up period, patients were assessed for response to treatment. Patients were considered a responder if they achieved both of the following:
- ≥30% improvement from baseline in the weekly average abdominal pain score based on the daily question: “In regards to your specific IBS symptoms of abdominal pain, on a scale of 0-10, what was your worst IBS-related abdominal pain over the last 24 hours? ‘Zero’ means you have no pain at all; ‘Ten’ means the worst possible pain you can imagine”.
- at least a 50% reduction in the number of days in a week with a daily stool consistency of Bristol Stool Scale type 6 or 7 compared with baseline where 6=fluffy pieces with ragged edges, a mushy stool; 7=watery stool, no solid pieces; entirely liquid.
Responders were then followed for recurrence of their IBS-related symptoms of abdominal pain or mushy/watery stool consistency for up to 20 treatment-free weeks.
When patients experienced recurrence of their symptoms of abdominal pain or mushy/watery stool consistency for 3 weeks of a rolling 4-week period, they were randomized into the double-blind, placebo-controlled repeat treatment phase. Of 1074 patients who responded to open-label XIFAXAN, 382 experienced a period of symptom inactivity or decrease that did not require repeat treatment by the time they discontinued, including patients who completed the 22 weeks after initial treatment with XIFAXAN. See Figure 3.
Overall, 1257 of 2579 patients (49%) were nonresponders in the open-label phase and per the study protocol were withdrawn from the study. Other reasons for discontinuation include: patient request (5%), patient lost to follow-up (4%), adverse reaction (3%), and other (0.8%).
There were 1074 (44%) of 2438 evaluable patients who responded to initial treatment with improvement in abdominal pain and stool consistency. The response rate for each IBS symptom during the open-label phase of Trial 3 is similar to the rates seen in Trials 1 and 2 (see Table 7). A total of 636 patients subsequently had sign and symptom recurrence and were randomized to the repeat treatment phase. The median time to recurrence for patients who experienced initial response during the open-label phase with XIFAXAN was 10 weeks (range 6 to 24 weeks).
The XIFAXAN (rifaximin) and placebo treatment groups had similar baseline IBS symptom scores at the time of recurrence and randomization to the double-blind phase, but symptom scores were less severe than at study entry into the open-label phase.
Patients were deemed to have recurrent signs and symptoms by the following criteria: a return of abdominal pain or lack of stool consistency for at least 3 weeks during a 4-week follow-up period. The primary endpoint in the double-blind, placebo-controlled portion of the trial was the proportion of patients who were responders to repeat treatment in both IBS-related abdominal pain and stool consistency as defined above during the 4 weeks following the first repeat treatment with XIFAXAN. The primary analysis was performed using the worst case analysis method where patients with <4 days of diary entries in a given week are considered as non-responders for that week.
More patients receiving XIFAXAN were monthly responders for abdominal pain and stool consistency in the primary analysis in Trial 3 (see Table 8).
Table 8. Efficacy Responder Rates in Trial 3 in a Given Week for at Least 2 Weeks During Weeks 3 to 6 of the Double-Blind, First Repeat Treatment Phase Placebo
(n=308)
n (%)
XIFAXAN
(n=328)
n (%)
Treatment
Difference
(95% CI)
Combined Responder: Abdominal Pain and Stool Consistency Responders
97 (31)
125 (38)
7%
(0.9%, 16.9%)
Abdominal Pain Responders (≥30% reduction in abdominal pain)
130 (42)
166 (51)
9%
(1.6%, 17.0%)
Stool Consistency Responders (≥50% reduction from baseline in days/week with loose or watery stools)
154 (50)
170 (52)
2%
(-4.7%, 11.0%)
Thirty six of 308 (11.7%) of placebo patients and 56 of 328 (17.1%) of XIFAXAN-treated patients responded to the first repeat treatment and did not have recurrence of signs and symptoms through the treatment-free follow-up period (10 weeks after first repeat treatment). The response rate difference was 5.4% with 95% confidence interval (1.2% to 11.6%).
-
15 REFERENCES
- 1. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard Ninth Edition. CLSI document M07-A9. Wayne, PA: Clinical and Laboratory Standards Institute, 2012.
- 2. Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria; Approved Standard Eighth Edition. CLSI document M11-A8. Wayne, PA: Clinical and Laboratory Standards Institute, 2012.
- 3. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement. CLSI document M100-S2. Wayne, PA: Clinical and Laboratory Standards Institute, 2014.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
The 200 mg tablet is a pink-colored, round, biconvex tablet with “Sx” debossed on one side and plain on the other. It is available in the following presentation:
-
- NDC: 65649-301-03, bottles of 30 tablets
The 550 mg tablet is a pink-colored, oval, biconvex tablet with “rfx” debossed on one side and plain on the other. It is available in the following presentations:
-
- NDC: 65649-303-02, bottles of 60 tablets
- NDC: 65649-303-03, carton of 60 tablets, Unit Dose
- NDC: 65649-303-04, carton of 42 tablets, Unit Dose
Storage
Store XIFAXAN Tablets at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
-
-
17 PATIENT COUNSELING INFORMATION
Persistent Diarrhea
For those patients being treated for travelers’ diarrhea, discontinue XIFAXAN if diarrhea persists more than 24-48 hours or worsens. Advise the patient to seek medical care for fever and/or blood in the stool [see Warnings and Precautions (5.1)].
Clostridium difficile-Associated Diarrhea
Clostridium difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including XIFAXAN, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibiotics alters the normal flora of the colon which may lead to C. difficile. Patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If diarrhea occurs after therapy or does not improve or worsens during therapy, advise patients to contact a physician as soon as possible [see Warnings and Precautions (5.2)].
Administration with Food
Inform patients that XIFAXAN may be taken with or without food.
Antibacterial Resistance
Counsel patients that antibacterial drugs including XIFAXAN should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When XIFAXAN is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by XIFAXAN or other antibacterial drugs in the future [see Warnings and Precautions (5.3)].
Distributed by:
Salix Pharmaceuticals, a division of
Bausch Health US, LLC
Bridgewater, NJ 08807 USA
Manufactured by:
Bausch Health Companies Inc.Steinbach, MB R5G 1Z7, Canada
Rifaximin for Travelers’ Diarrhea, Hepatic Encephalopathy and IBS are protected by U.S. Patent Nos. 7,045,620; 7,612,199; 7,902,206; 7,906,542; 8,158,781; 8,158,644; 8,193,196; 8,518,949; 8,741,904; 8,835,452; 8,853,231 and 9,271,968. Rifaximin for Travelers’ Diarrhea is also protected by U.S. Patent No. 7,928,115. Rifaximin for Hepatic Encephalopathy is also protected by U.S. Patent No. 8,642,573; 8,829,017; 8,946,252; 8,969,398; 9,421,195; and 9,629,828; 10,314,828 and 10,335,397. Rifaximin for IBS is also protected by U.S. Patent Nos. 7,915,275 and 8,309,569.
The Xifaxan 200 mg and 550 mg products and the Xifaxan trademark are licensed by Alfasigma S.p.A. to Salix Pharmaceuticals or its affiliates.
All other product/brand names are trademarks of the respective owners.
© 2019 Salix Pharmaceuticals, Inc. or its affiliates
Website: www.Salix.com
9693700
20002738 - PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
- Package/Label Display Panel
-
INGREDIENTS AND APPEARANCE
XIFAXAN
rifaximin tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 65649-301 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RIFAXIMIN (UNII: L36O5T016N) (RIFAXIMIN - UNII:L36O5T016N) RIFAXIMIN 200 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERYL PALMITOSTEARATE (UNII: GSY51O183C) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) FERRIC OXIDE RED (UNII: 1K09F3G675) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color PINK (PINK) Score no score Shape ROUND (BICONVEX) Size 10mm Flavor Imprint Code Sx Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65649-301-05 10 in 1 CARTON 07/25/2004 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC: 65649-301-03 30 in 1 BOTTLE; Type 0: Not a Combination Product 07/25/2004 3 NDC: 65649-301-41 100 in 1 BOTTLE; Type 0: Not a Combination Product 07/25/2004 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021361 07/25/2004 XIFAXAN
rifaximin tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 65649-303 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RIFAXIMIN (UNII: L36O5T016N) (RIFAXIMIN - UNII:L36O5T016N) RIFAXIMIN 550 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) GLYCERYL PALMITOSTEARATE (UNII: GSY51O183C) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) FERRIC OXIDE RED (UNII: 1K09F3G675) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color PINK (PINK) Score no score Shape OVAL (OVAL) Size 19mm Flavor Imprint Code rfx Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65649-303-03 6 in 1 CARTON 05/01/2010 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC: 65649-303-02 60 in 1 BOTTLE; Type 0: Not a Combination Product 05/01/2010 3 NDC: 65649-303-04 14 in 1 CARTON 05/01/2010 04/30/2020 3 3 in 1 BLISTER PACK; Type 0: Not a Combination Product 4 NDC: 65649-303-01 3 in 1 TRAY 05/01/2010 4 1 in 1 CARTON 4 2 in 1 BLISTER PACK; Type 0: Not a Combination Product 5 NDC: 65649-303-05 3 in 1 TRAY 05/01/2010 5 1 in 1 CARTON 5 3 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021361 05/01/2010 Labeler - Salix Pharmaceuticals, Inc. (793108036) Establishment Name Address ID/FEI Business Operations Patheon Inc. 205475333 MANUFACTURE(65649-301, 65649-303) Establishment Name Address ID/FEI Business Operations Alfasigma SPA 438537978 MANUFACTURE(65649-303) Establishment Name Address ID/FEI Business Operations Carton Service, Incorporated 928861723 REPACK(65649-301, 65649-303) , RELABEL(65649-301, 65649-303) , LABEL(65649-301, 65649-303) , PACK(65649-301, 65649-303) Establishment Name Address ID/FEI Business Operations Bausch Health Companies Inc. 253292734 MANUFACTURE(65649-301, 65649-303)
Trademark Results [XIFAXAN]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 XIFAXAN 78428755 2965332 Live/Registered |
ALFASIGMA S.P.A. 2004-06-02 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.