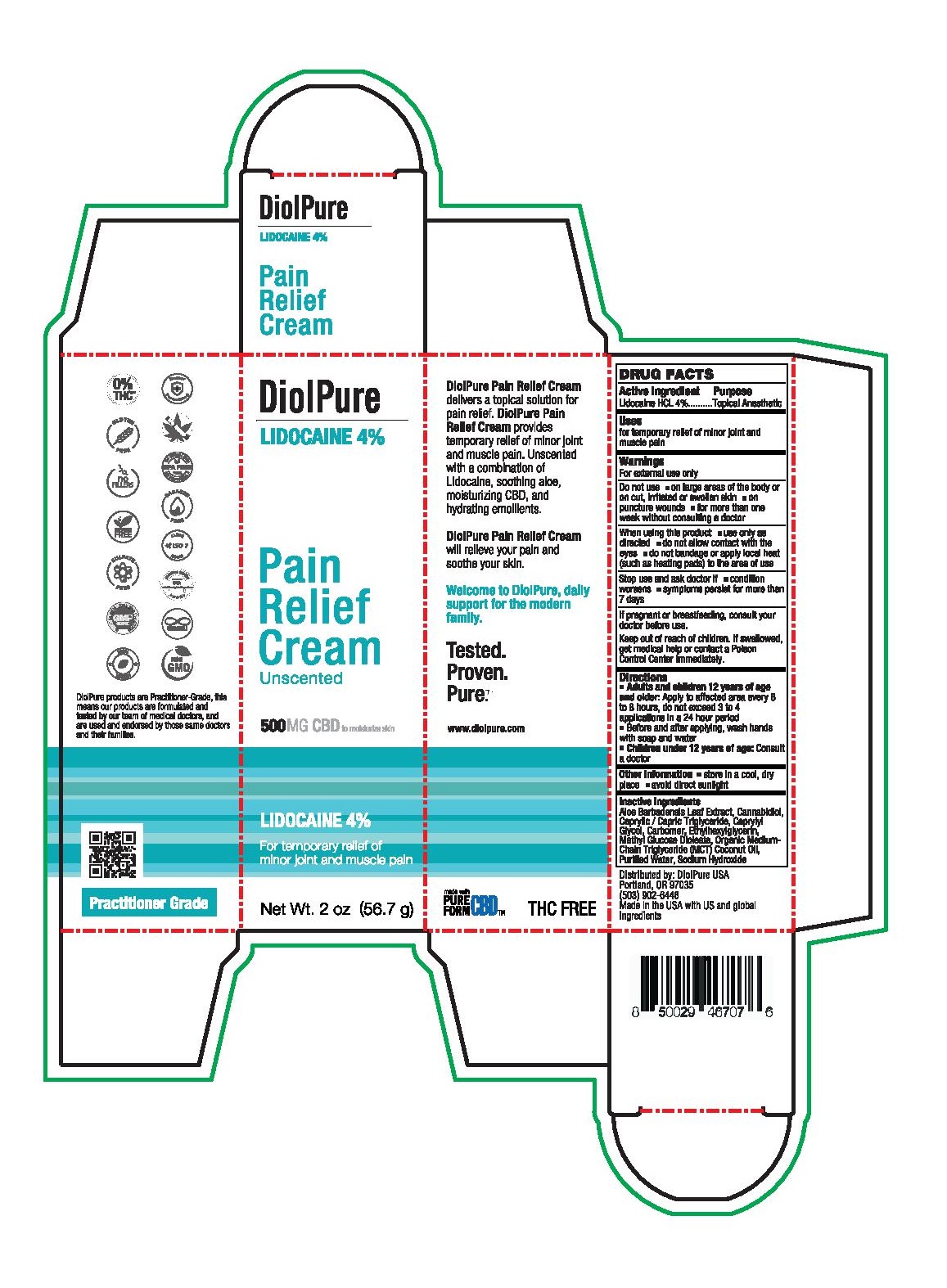
DiolPure Lidocaine Pain Relief Cream
DiolPure Lidocaine Pain Relief Cream by
Drug Labeling and Warnings
DiolPure Lidocaine Pain Relief Cream by is a Otc medication manufactured, distributed, or labeled by PureForm Global, BioLyte Laboratories, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
DIOLPURE LIDOCAINE PAIN RELIEF CREAM- lidocaine hcl cream
PureForm Global
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
DiolPure Lidocaine Pain Relief Cream
Warnings
For external use only
Do not use
- on large areas of the body or on cut, irritated or swollen skin
- on puncture wounds
- for more than one week without consulting a doctor
Directions
- Adults and children 12 years of age and older: Apply to affected area every 6 to 8 hours, do not exceed 3 to 4 applications in a 24 hour period
- Before and after applying, wash hands with soap and water
- Children under 12 years of age: Consult a doctor
| DIOLPURE LIDOCAINE PAIN RELIEF CREAM
lidocaine hcl cream |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - PureForm Global (081350045) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BioLyte Laboratories, LLC | 015560564 | manufacture(73439-701) | |
Revised: 7/2021
Document Id: c6a8fe27-3703-1cc0-e053-2995a90af71a
Set id: c6148367-8bd5-7372-e053-2a95a90a27fc
Version: 2
Effective Time: 20210708
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.