Tender Moments Delicate Baby Bottom Balm
Tender Moments Delicate Baby Bottom Balm by
Drug Labeling and Warnings
Tender Moments Delicate Baby Bottom Balm by is a Otc medication manufactured, distributed, or labeled by Jafra Cosmetics International, Distribuidora Comercial Jafra, S.A. de C.V.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
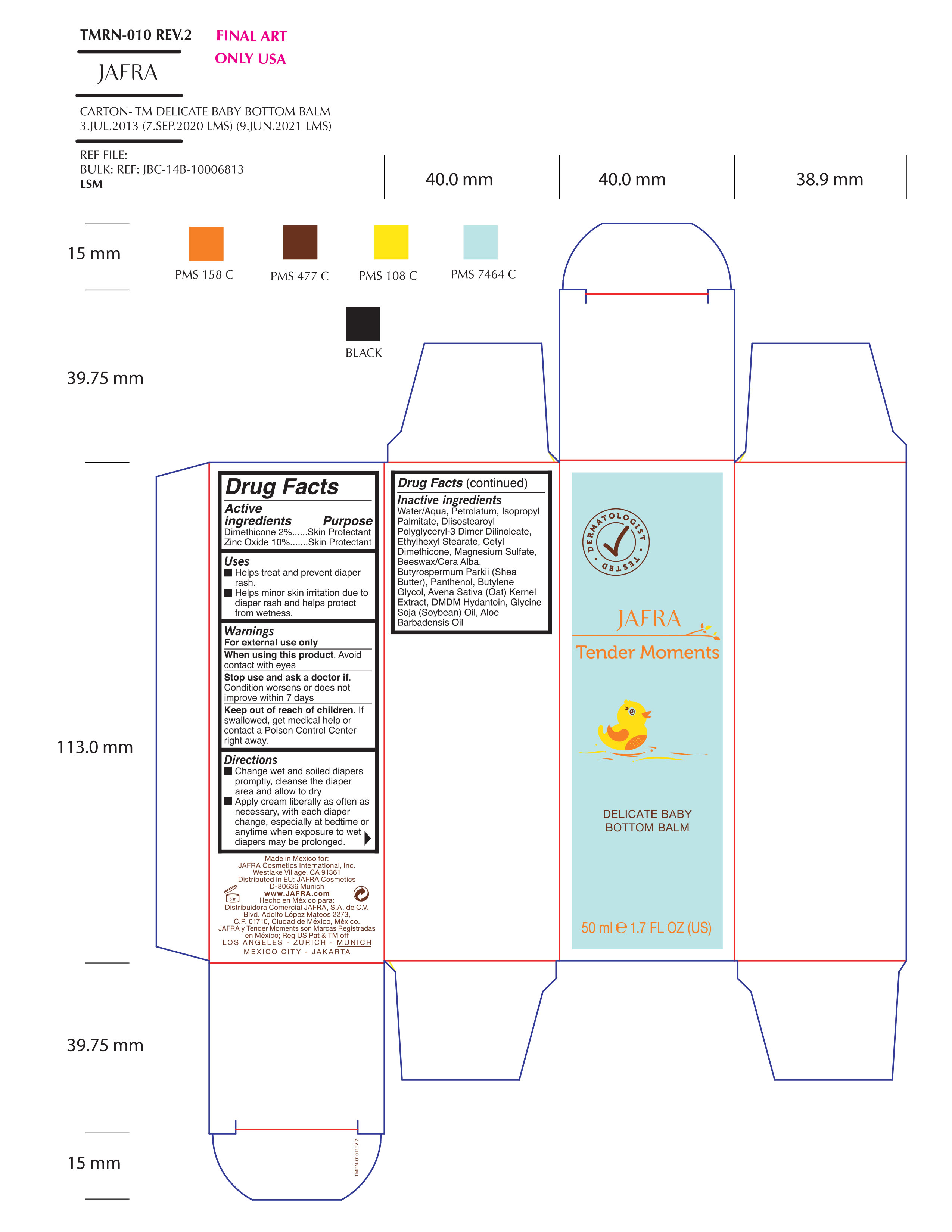
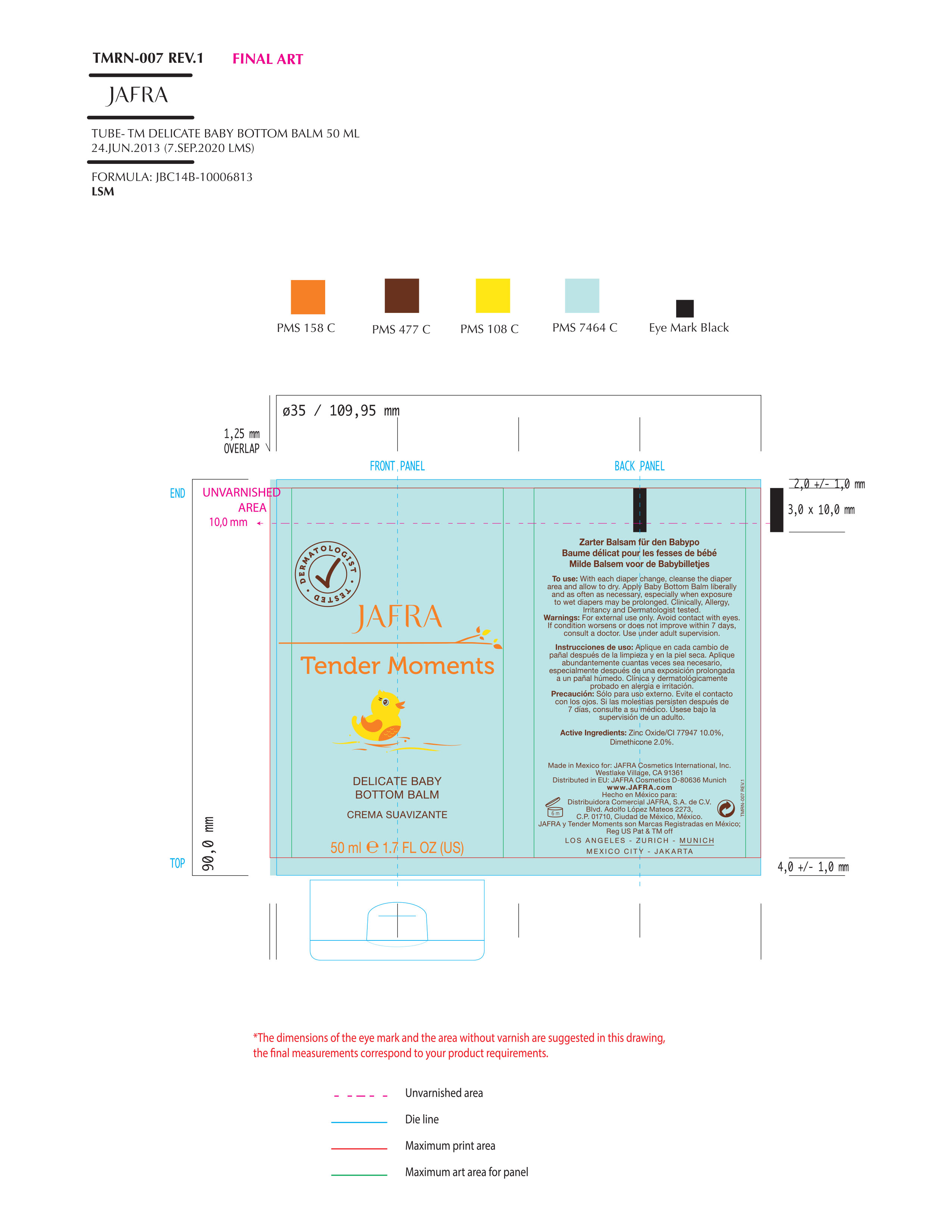
TENDER MOMENTS DELICATE BABY BOTTOM BALM- dimethicone, zinc oxide cream
Jafra Cosmetics International
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Tender Moments Delicate Baby Bottom Balm
Uses
- Helps treat and prevent diaper rash
- Helps Minor skin irritation due to diaper rash and helps protect from wetness
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Change wet and soiled diapers promptly, cleanse the diaper area and allow to dry
- Apply cream liberally as often as necessary, with each diaper change, especially at bedtime or anytime when exposure to wet diapers may be prolongued.
Inactive ingredients
Water/Aqua, Petrolatum, Isopropyl Palmitate, Diisostearoyl Polyglyceryl-3 Dimer Dilinoleate, Ethylhexyl Stearate, Cetyl Dimethicone, Magnesium Sulfate, Beeswax/Cera Alba, Butyrospermum Parkii (Shea Butter), Panthenol, Butylene Glycol, Avena Sativa (Oat) Kernel Extract, DMDM Hydantoin, Glycine Soja (Soybean) Oil, Aloe Barbadensis Oil
| TENDER MOMENTS DELICATE BABY BOTTOM BALM
dimethicone, zinc oxide cream |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Jafra Cosmetics International (041676479) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Distribuidora Comercial Jafra, S.A. de C.V. | 951612777 | manufacture(68828-298) | |