Allergy Relief by CHAIN DRUG CONSORTIUM 1195A-PRV-2020-0903
Allergy Relief by
Drug Labeling and Warnings
Allergy Relief by is a Otc medication manufactured, distributed, or labeled by CHAIN DRUG CONSORTIUM. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ALLERGY RELIEF- loratadine capsule, liquid filled
CHAIN DRUG CONSORTIUM
----------
1195A-PRV-2020-0903
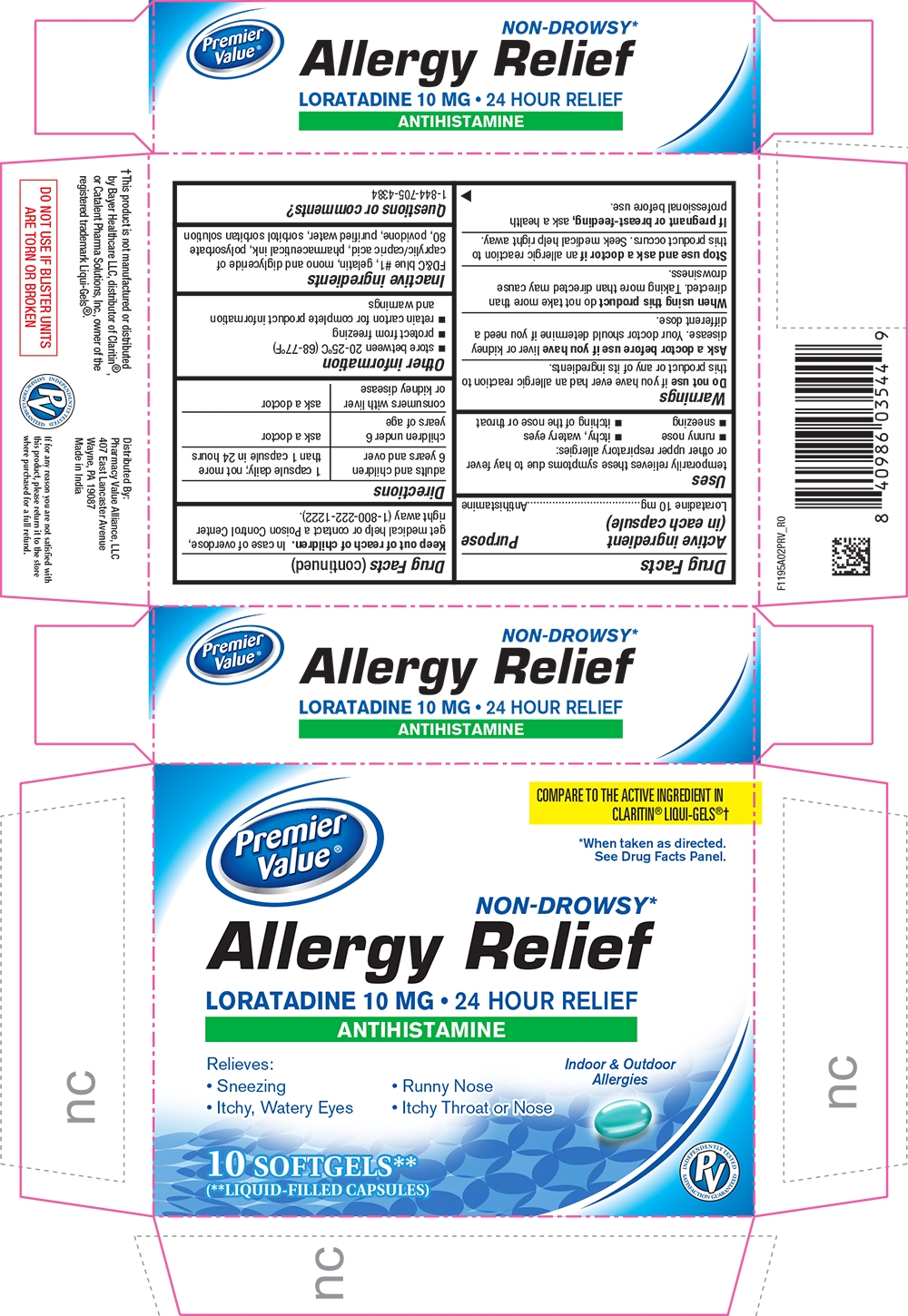
Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies
runny nose
sneezing
itchy, water eyes
itching of the nose or throat
Warnings
Do not use if you have ever had an allergic reaction to this product or any of its ingredients.
Ask a doctor before use if you have liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product do not take more than directed. Taking more than directed may cause drowsiness.
Directions
| adults and children 6 years and over | 1 capsule daily; not more than 1 capsule in 24 hours |
| children under 6 years of age | ask a doctor |
| consumers with liver or kidney disease | ask a doctor
|
Other information
Store between 20-25˚C (68-77˚F)
protect from freezing
retain carton for complete information and warnings
Inactive ingredients
FD&C Blue no. 1, gelatine, mono anddiglyceride of caprilic/capric acid, pharmaceutical ink, polysorbate 80,povidone, purified water, sorbitol, sorbitan solution.
PRINCIPAL DISPLAY PANEL
Premier Value®
COMPARE TO THE ACTIVE INGREDIENT IN CLARITIN® LIQUI-GELS®†
*When taken as directed.
See Drug Facts Panel.
NON-DROWSY*
Allergy Relief
LORATADINE 10 MG 24 HOUR RELIEF
ANTIHISTAMINE
Relieves:
Sneezing
Runny Nose
Itchy, Watery Eyes
Itchy Throar or Nose
Indoor Outdoor Allergies
10 SOFTGELS**
(**LIQUID-FILLED CAPSULES)
| ALLERGY RELIEF
loratadine capsule, liquid filled |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - CHAIN DRUG CONSORTIUM (101668460) |
Trademark Results [Allergy Relief]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ALLERGY RELIEF 98236984 not registered Live/Pending |
Dmytro Kononenko 2023-10-24 |
 ALLERGY RELIEF 90457167 not registered Live/Pending |
American Textile Company, Inc. 2021-01-10 |
 ALLERGY RELIEF 78838437 3358249 Live/Registered |
Meshbesher Health Corporation 2006-03-16 |
 ALLERGY RELIEF 76619855 3066888 Live/Registered |
AMERICAN TEXTILE COMPANY 2004-11-09 |
 ALLERGY RELIEF 74668018 not registered Dead/Abandoned |
NaturaLife Corporation 1995-05-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.