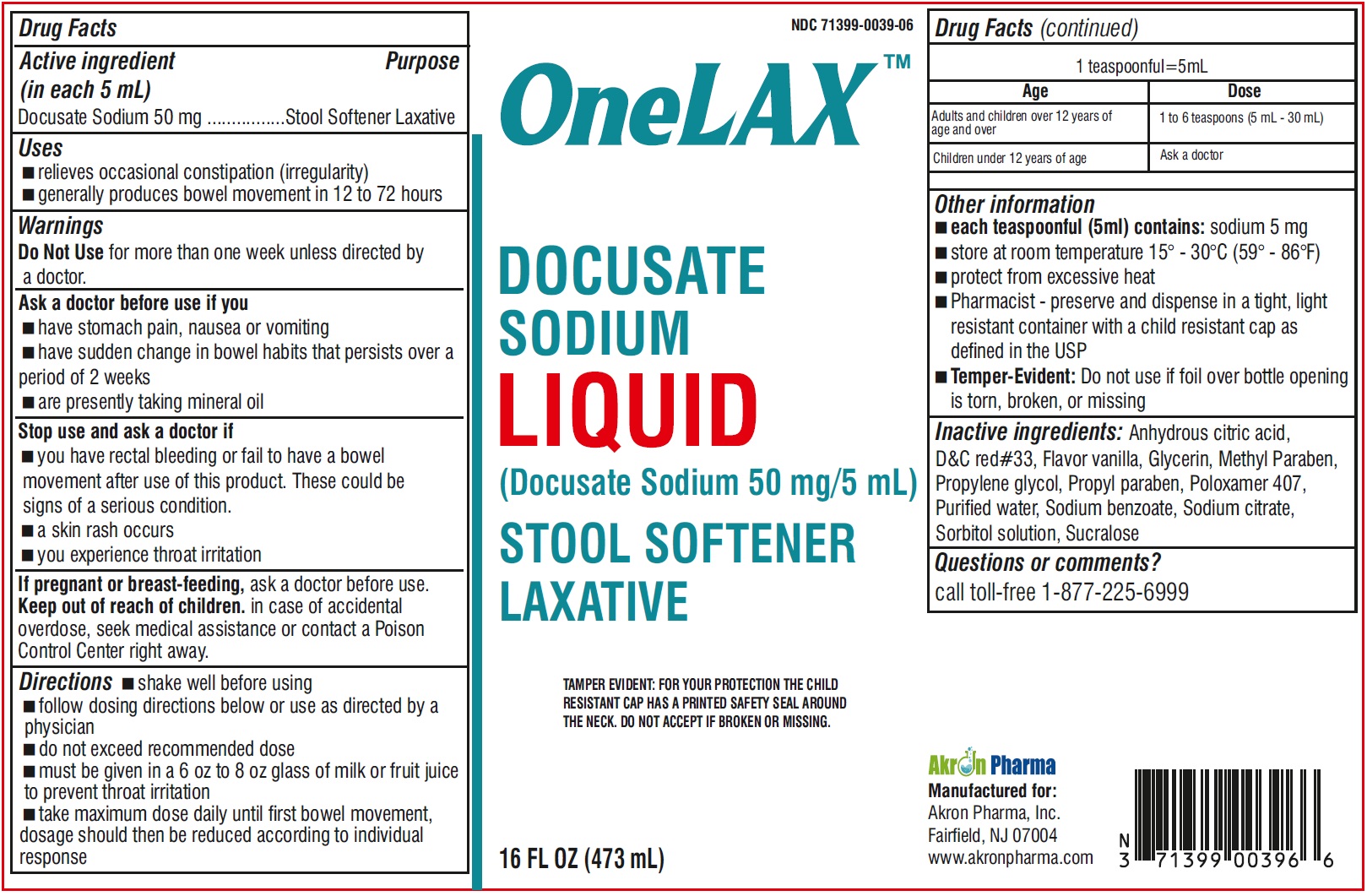
OneLAXDocusate Sodium Liquid50 mg/5 mL
OneLAX by
Drug Labeling and Warnings
OneLAX by is a Otc medication manufactured, distributed, or labeled by Akron Pharma. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ONELAX- docusate sodium liquid
Akron Pharma
----------
OneLAX
Docusate Sodium Liquid
50 mg/5 mL
Uses
- relieves occasional constipation (irregularity)
- generally produces bowel movement in 12 to 72 hours
Ask a doctor before use if you
- have stomach pain, nausea or vomiting
- have sudden change in bowel habits that persists over a period of 2 weeks
- are presently taking mineral oil
Stop use and ask a doctor if
- you have rectal bleeding or fail to have a bowel movement after use of this product. These could be signs of a serious condition.
- a skin rash occurs
- you experience throat irritation
Keep out of reach of children
In case of accidental overdose, seek medical assistance or contact a Poison Control Center right away.
Directions
- shake well before using
- follow dosing directions below or use as directed by a physician
- do not exceed recommended dose
- must be given in a 6 oz to 8 oz glass of milk or fruiit juice to prevent throat irritation
- take maximum dose daily until first bowel movement, dosage should then be reduced according to indivisual response
| Age | Dose |
| Adults and children over 12 years of age and over | 1 to 6 teaspoons (5 mL - 30 mL) |
| Children under 12 years of age | Ask a doctor |
Other information
- wach teaspoonful (5 ml) contains: sodium 5 mg
- store at room temperature 15o - 30oC (59o - 86oF)
- protect from excessive heat
- Pharmacist-preserve and dispense in a tight, light resistant container with a child resistant cap as defined in the USP
- Temper -Evident: Do not use if foil over bottle opening is torn, broken, or missing
Inactive ingredients:
Anhydrous citric acid, D&C red#33, Flavor vanilla, Glycerin, Methyl Paraben, Propylene glycol, Propyl paraben, Poloxamer 407, Purified water, Sodium benzoate, Sodium citrate, Sorbitol solution, Sucralose
| ONELAX
docusate sodium liquid |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Akron Pharma (067878881) |
Revised: 1/2025
<
Document Id: fcdbfa53-1565-4d9a-a100-841e018de4d6
Set id: c692c99c-6fc8-42fb-bd8a-25ed4979d70b
Version: 2
Effective Time: 20250105
Trademark Results [OneLAX]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ONELAX 98064007 not registered Live/Pending |
AKRON GENERICS LLC 2023-06-29 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.