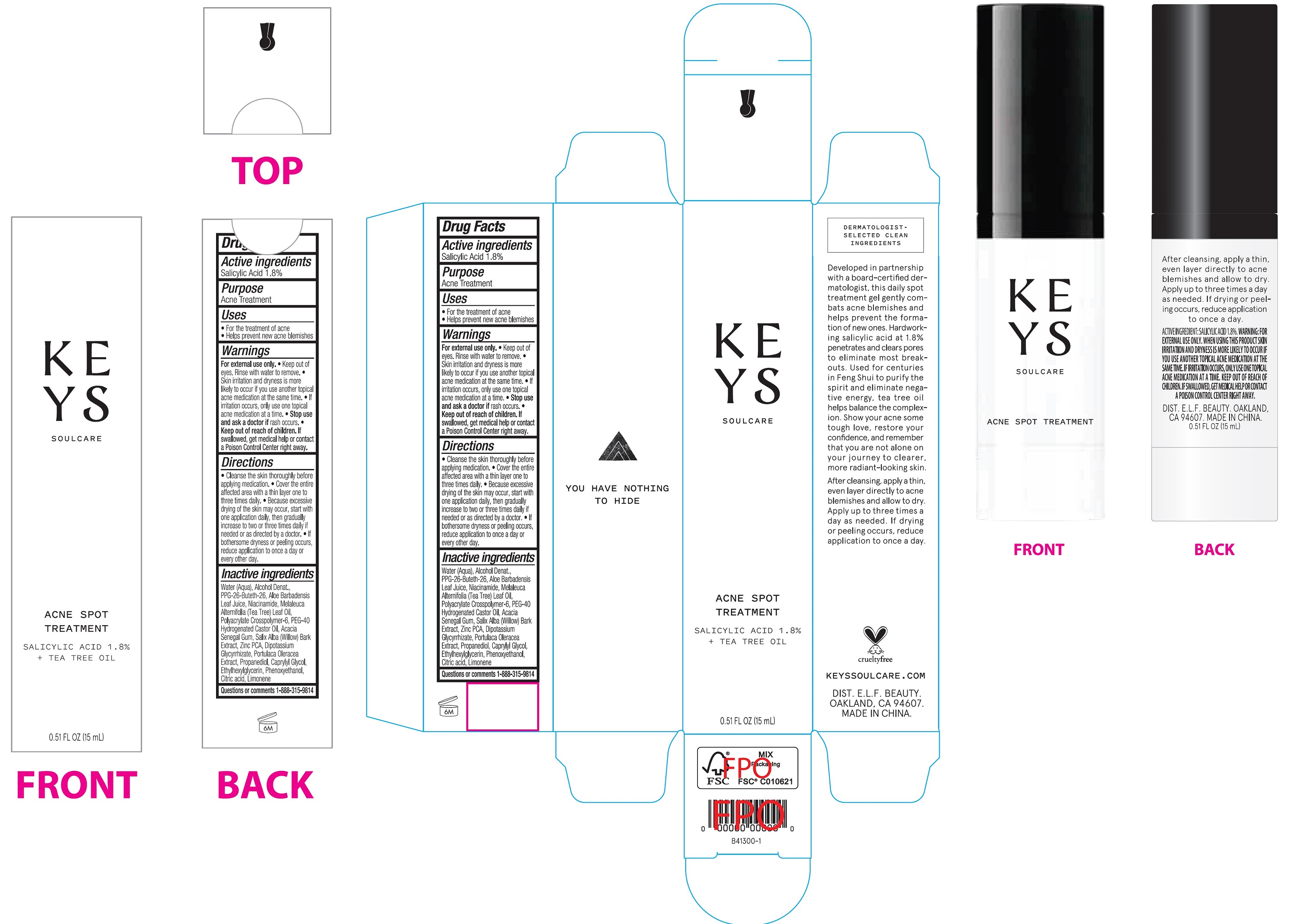
Acne Spot Treatment by e.l.f. Cosmetics, Inc Acne Spot Treatment
Acne Spot Treatment by
Drug Labeling and Warnings
Acne Spot Treatment by is a Otc medication manufactured, distributed, or labeled by e.l.f. Cosmetics, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ACNE SPOT TREATMENT- salicylic acid liquid
e.l.f. Cosmetics, Inc
----------
Acne Spot Treatment
Warnings
For external use only.
- Keep out of eyes. Rinse with water to remove.
- Skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time.
- If irritation occurs, only use one topical acne medication at a time.
Directions
- Cleanse the skin thoroughly before applying medication.
- Cover the entire affected area with a thin layer one to three times daily.
- Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if neede or a directed by a doctor.
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
Inactive Ingredients
Water (Aqua), Alcohol Denat., PPg-26-Buteth-26, Aloe Barbadensis Leaf Juice, Niacinamide, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Polyacrylate Crosspolymer-6, PEG-40 Hydrogenated Castor Oil, Acacia Seneggl Gum, Salix Alba (Willow) Bark Extract, Zinc PCA, Dipotassium Glycyrrhizate, Portulaca Oleracea Extract, Propanediol, Caprylyl glycol, Ethylhexylglycerin, Phenoxyethanol, Citric acid, Limonene
| ACNE SPOT TREATMENT
salicylic acid liquid |
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Labeler - e.l.f. Cosmetics, Inc (093902816) |
Revised: 11/2024
Document Id: 26416a04-2911-80cd-e063-6394a90abc9a
Set id: c716ab24-4ee0-197a-e053-2995a90a3a99
Version: 5
Effective Time: 20241106
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.