FLUTICARE NASAL ALERGY (fluticasone propionate- glucocorticoid spray, metered
FlutiCare by
Drug Labeling and Warnings
FlutiCare by is a Otc medication manufactured, distributed, or labeled by Innovus Pharmaceuticals Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient (in each spray)
- Purpose
- Uses
-
Warnings
Only for use in the nose. Do not spray into your eyes or mouth.
Do not use
- in children under 4 years of age
- to treat asthma
- if you have an injury or surgery to your nose that is not fully healed
- if you have ever had an allergic reaction to this product or any of the ingredients
Ask a doctor before use if you have or had
glaucoma or cataracts
Ask a doctor or pharmacist before use if you are taking
- medicine for HIV infection (such as ritonavir)
- a steroid medicine for asthma, allergies or skin rash
- ketoconazole pills (medicine for fungal infection)
When using this product
- the growth rate of some children may be slower
- stinging or sneezing may occur for a few seconds right after use
- do not share this bottle with anyone else as this may spread germs
- remember to tell your doctor about all the medicines you take, including this one
Stop use and ask a doctor if
- you have, or come into contact with someone who has, chicken pox, measles or tuberculosis
- your symptoms do not get better within 7 days of starting use or you get new symptoms such as severe facial pain or thick nasal discharge. You may have something more than allergies, such as an infection.
- you get a constant whistling sound from your nose. This may be a sign of damage inside your nose.
- you get an allergic reaction to this product. Seek medical help right away.
- you get new changes to your vision that develop after starting this product
- you have severe or frequent nosebleeds
If pregnant or breast-feeding,
- ask a health professional before use.
- Keep Out of Reach of Children
-
Directions
-
read the Instructions for Use for how to:
- o prime the bottle
- o use the spray
- o clean the spray nozzle
- shake gently before each use
- use this product only once a day
- do not use more than directed
ADULTS AND CHILDREN 12 YEARS OF AGE AND OLDER
- Week 1 – use 2 sprays in each nostril once daily
- Week 2 through 6 months – use 1 or 2 sprays in each nostril once daily, as needed to treat your symptoms
- After 6 months of daily use – ask your doctor if you can keep using
CHILDREN 4 TO 11 YEARS OF AGE
- the growth rate of some children may be slower while using this product. Children should use for the shortest amount of time necessary to achieve symptom relief. Talk to your child’s doctor if your child needs to use the spray for longer than two months a year.
- an adult should supervise use
- use 1 spray in each nostril once daily
CHILDREN UNDER 4 YEARS OF AGE
- do not use
-
read the Instructions for Use for how to:
- Other information
- Inactive Ingredients
- Questions or comments?
-
Instructions For Use
FlutiCare Allergy Symptom Reliever Nasal Spray
Fluticasone propionate (glucocorticoid) 50 mcg per spray
(FLOO-tih-kah-sone)
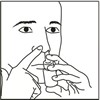
FlutiCare nasal spray is for use in your nose only.
Read this information before you start using your FlutiCare nasal spray.
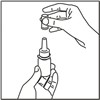
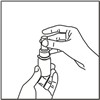
Your FlutiCare nasal spray must be primed before you use it for the first time and when you have not used it for a week or more.
How to prime your FlutiCare nasal spray:
Do not use this bottle for more than the labeled number of sprays even though the bottle is not completely empty.
Cleaning your FlutiCare nasal spray:
Your nasal spray should be cleaned at least 1 time each week.
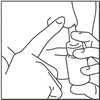
- 1. Remove the dust cap and then gently pull upwards to free the nasal applicator.
- 2. Wash the applicator and dust cap under warm tap water. Allow to dry at room temperature.
- 3. Place the applicator and dust cap back on the bottle.
- 4. If the nasal applicator becomes blocked, it can be removed and left to soak in warm water. Rinse the nasal applicator with cold tap water. Dry the nasal applicator and place it back on the bottle. Do not try to unblock the nasal applicator by inserting a pin or other sharp object.
Storing your FlutiCare nasal spray:
- ➢ Store between 39° and 86°F (4° and 30°C).
- ➢ Do not use your FlutiCare nasal spray after the date shown as “EXP” on the label or box.
Distributed by:
Innovus Pharmaceuticals, Inc.
San Diego, CA 92122Revised September 2017
062017/00 - Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
FLUTICARE NASAL ALERGY
fluticasone propionate (glucocorticoid) spray, meteredProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 57483-005 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLUTICASONE PROPIONATE (UNII: O2GMZ0LF5W) (FLUTICASONE - UNII:CUT2W21N7U) FLUTICASONE PROPIONATE 50 ug Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) PHENYLETHYL ALCOHOL (UNII: ML9LGA7468) POLYSORBATE 80 (UNII: 6OZP39ZG8H) WATER (UNII: 059QF0KO0R) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 57483-005-02 1 in 1 CARTON 09/22/2017 1 120 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA207957 09/22/2017 Labeler - Innovus Pharmaceuticals Inc. (962507187) Registrant - Innovus Pharmaceuticals Inc. (962507187)
Trademark Results [FlutiCare]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 FLUTICARE 98371336 not registered Live/Pending |
Yanggen, Steven Douglas 2024-01-23 |
 FLUTICARE 87601188 5471512 Live/Registered |
Innovus Pharmaceuticals, Inc. 2017-09-08 |
 FLUTICARE 87262502 5331309 Live/Registered |
Innovus Pharmaceuticals, Inc. 2016-12-08 |
 FLUTICARE 85971232 not registered Dead/Abandoned |
Novalere FP, Inc. 2013-06-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.