PRIMOR- sulfadimethoxine and ormetoprim tablet
Primor by
Drug Labeling and Warnings
Primor by is a Animal medication manufactured, distributed, or labeled by Zoetis Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- WARNINGS
-
DESCRIPTION
DESCRIPTION: Primor is an antimicrobial drug containing sulfadimethoxine and ormetoprim in a 5 to 1 ratio. The combination of these 2 compounds results in the potentiation of sulfadimethoxine, providing increased efficacy, a broadened spectrum of activity to include some sulfonamide-resistant organisms, and reduction in the rate of resistance development.
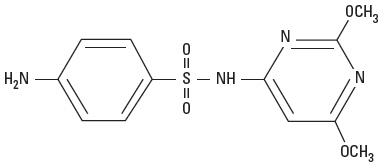
Sulfadimethoxine is a white, almost tasteless and odorless powder. Chemically, it is N1-(2,6-dimethoxy-4-pyrimidinyl)-sulfanila-mide. The structural formula is:
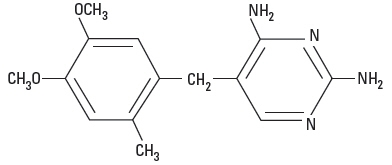
Ormetoprim is a white, almost tasteless powder. Chemically, it is 2,4-diamino-5-(4,5-dimethoxy-2-methylbenzyl)-pyrimidine. The structural formula is:
-
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGY: Sulfadimethoxine is not acetylated in the dog, as in most other animals, and is excreted predominantly as the unchanged drug.3 Sulfadimethoxine has a relatively high solubility at the pH normally occurring in the kidney, precluding the possibility of precipitation and crystalluria. Slow renal excretion results from a high degree of tubular reabsorption. Plasma protein binding is very high, providing a blood reservoir of the drug. Thus sulfadimethoxine maintains higher blood levels than most other long-acting sulfonamides. Single, comparatively low doses of sulfadimethoxine give rapid and sustained therapeutic blood levels.4
The systemically active sulfonamides, which include sulfadimethoxine, are bacteriostatic agents. Sulfonamides competitively inhibit bacterial synthesis of folic acid (pteroylglutamic acid) from para-aminobenzoic acid. Mammalian cells are capable of utilizing folic acid in the presence of sulfonamides.
Ormetoprim, like other diaminopyrimidines, inhibits the reduction of dihydrofolic acid to tetrahydrofolic acid by bacterial cells.
Sulfadimethoxine/ormetoprim thus blocks 2 sequential steps of the folic acid metabolism of bacteria, depriving them of folate coenzymes. Potentiated sulfonamides have been shown to exhibit bactericidal as well as bacteriostatic action.
Microbiology: Sulfadimethoxine is a low-dosage, rapidly absorbed, long-acting sulfonamide effective for the treatment of a wide range of bacterial infections commonly encountered in dogs. Sulfadimethoxine has been demonstrated under laboratory and field conditions to be effective against a variety of gram-positive and gram-negative, aerobic and anaerobic organisms belonging to the genera Streptococcus, Klebsiella, Proteus, Shigella, Staphylococcus, Escherichia, Salmonella, and Clostridium.1,2 Most strains of these organisms were found to be susceptible to Primor in vitro, but the in vivo significance has not been determined for some canine isolates.
Ormetoprim potentiates the activity of sulfadimethoxine. The in vitro antibacterial spectrum and activity of the 2 compounds are very similar. On a molar basis, ormetoprim is more active than sulfadimethoxine. Sulfadimethoxine/ormetoprim shows enhanced in vitro and in vivo activity (potentiation) over that of either compound used alone. In vitro, this potentiation results in a reduction of the minimum inhibitory concentration of each drug, and an increase in activity against sulfonamide-resistant organisms, such as Streptococcus, Staphylococcus, Corynebacterium, Escherichia, Klebsiella, Proteus, Brucella, Bordetella, and Clostridium.1 The susceptibility of organisms to Primor Tablets should be determined using a potentiated sulfonamide sensitivity disc such as sulfa-methoxazole and trimethoprim (BBL® Sensi-Disc® SXT1). Specimens for susceptibility testing should be collected prior to initiation of therapy.
In an experimentally induced, controlled soft tissue infection study in dogs, the therapeutic efficacy of Primor was significantly greater than the 2 individual components when administered separately, providing clear evidence of the potentiation of sulfa-dimethoxine by ormetoprim in the target species.1
- 1 BBL® and Sensi-Disc® are registered trademarks owned by Becton, Dickinson and Company, Paramus, New Jersey.
Blood Levels:1 Therapeutically effective blood levels of both sulfadimethoxine and ormetoprim are obtained and maintained in dogs when using the recommended Primor dosing regimen of 25 mg/lb on day one and of 12.5 mg/lb on following days. Blood levels of sulfadimethoxine and ormetoprim were studied in 2 male and 2 female dogs. The initial drug dose was administered at zero hours. Blood samples were taken at 11 intervals, 6 of which are reported in Table 1. A second dose of Primor was administered immediately following the 24-hour blood samples and blood values were determined 2 hours later (26 hours from the initial dose).
Table 1. Blood levels (mcg/mL) obtained with administration of a 25 mg/lb dose of Primor followed with a 12.5 mg/lb dose at 24 hours Sample 2 hr 8 hr 24 hr* 26 hr 32 hr 48 hr - * Sample collected before administration of the second dose.
Sulfadimethoxine 23.00 41.00 39.00 60.00 67.00 36.00 Ormetoprim 1.04 0.55 0.09 1.08 0.44 0.03 -
INDICATIONS & USAGE
INDICATIONS AND USAGE: Primor is for the treatment of skin and soft tissue infections (wounds and abscesses) in dogs caused by strains of Staphylococcus aureus and Escherichia coli and urinary tract infections caused by Escherichia coli, Staphylococcus spp., and Proteus mirabilis susceptible to sulfadimethoxine/ormetoprim.
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
PRECAUTIONS: Decreased water consumption and aciduria enhance the probability of the formation of sulfonamide crystals in the urine. Monitoring urine samples for crystal formation is recommended from animals with acid urine receiving the drug. As with any sulfonamide therapy, make certain dogs maintain adequate water intake. If dogs show no improvement within 2 or 3 days, reevaluate the diagnosis.
Safety in breeding dogs has not been established.
-
ADVERSE REACTIONS
ADVERSE REACTIONS: Conditions reported following use of sulfonamides or potentiated sulfonamides include polyarthritis, urticaria, facial swelling, fever, hemolytic anemia, polydypsia, polyuria, hepatitis, vomiting, anorexia, diarrhea, and neurologic disorders. In rare instances, neurologic signs including behavioral changes, ataxia, seizures, aggression, and hyperexcitability have been reported. Keratitis sicca, possibly due to prolonged use of sulfonamides, has been reported.
Individual animal hypersensitivity may result in local or generalized reactions. Anaphylactoid reactions, although rare, may also occur.
Antidote: Epinephrine for anaphylactoid reactions.
-
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION: Administer an initial oral dose of 25 mg/lb (55 mg/kg) of body weight on the first day of treatment. Administer subsequent daily doses at the rate of 12.5 mg/lb (27.5 mg/kg) of body weight. Continue treatment for at least 2 days after remission of clinical signs. Do not extend treatment for more than 21 consecutive days. Suggested dosage schedules follow:
Body Weight (lb)
Up ToNo. of Tablets
First DayNo. of Tablets
Subsequent DaysPrimor 120 5 1 1/2 10 2 1 15 3 1 1/2 Primor 240 10 1 1/2 20 2 1 30 3 1 1/2 Primor 600 25 1 1/2 50 2 1 Primor 1200 50 1 1/2 100 2 1 For optimal therapeutic effect: (1) the drug must be given early in the course of the disease; (2) therapeutically effective levels must be maintained in the body throughout the treatment period; (3) treatment should continue for at least 2 days after remission of clinical signs; and (4) the causative bacterial agents must be sensitive to the drug.
-
ANIMAL PHARMACOLOGY & OR TOXICOLOGY
TOXICITY AND SAFETY:1 Toxicity data for Primor indicate that the drug is safe when used at the recommended dosage.
Following oral administration of Primor to dogs at 27.5 mg/kg/day (12.5 mg/lb/day) for 8 weeks, no changes were noted in hematology, blood chemistry, urinalysis, gross pathology, and histopathology, except for elevated serum cholesterol, increased thyroid and liver weights, enlarged basophilic cells in the pituitary, and mild follicular thyroid hyperplasia. These changes are known to be associated with prolonged administration of sulfonamides to dogs and have been shown to be reversible.
- STORAGE AND HANDLING
-
HOW SUPPLIED
HOW SUPPLIED: Primor is available as scored tablets for the following potencies: 120 mg, 240 mg, 600 mg, and 1200 mg.
PRIMOR 120:
100 mg sulfadimethoxine/20 mg ormetoprimPRIMOR 600:
500 mg sulfadimethoxine/100 mg ormetoprimPRIMOR 240:
200 mg sulfadimethoxine/40 mg ormetoprimPRIMOR 1200:
1000 mg sulfadimethoxine/200 mg ormetoprim -
REFERENCES:
1. Data on file, Pfizer Animal Health.
2. Bevill RF: Sulfonamides. Booth NM, MacDonald LE (eds), Veterinary Pharmacology and Therapeutics, 5th ed, The Iowa State University Press, Ames, Iowa, chapter 48, 1982.
3. Bridges JW, Kirby MR, Walker SR, et al: Species differences in the metabolism of sulfadimethoxine. Biochem J 109:851, 1968.
4. Baggot JD: Some aspects of drug persistence in domestic animals. Res Vet Sci 11(2):130, 1970.
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 120 mg Tablet Bottle Label
PRIMOR® 120
(sulfadimethoxine/ormetoprim)Each tablet contains 100 mg sulfadimethoxine
and 20 mg ormetoprim.For use in dogs only
Not for Human Use
Keep Out of Reach of Children
Caution: Federal law restricts this drug to use
by or on the order of a licensed veterinarian.100 tablets
NADA #100-929, Approved by FDAzoetis

-
PRINCIPAL DISPLAY PANEL - 240 mg Tablet Bottle Label
PRIMOR® 240
(sulfadimethoxine/ormetoprim)Each tablet contains 200 mg sulfadimethoxine
and 40 mg ormetoprim.For use in dogs only
Not for Human Use
Keep Out of Reach of Children
Caution: Federal law restricts this drug to use
by or on the order of a licensed veterinarian.100 tablets
NADA #100-929, Approved by FDAzoetis
-
PRINCIPAL DISPLAY PANEL - 600 mg Tablet Bottle Label
PRIMOR® 600
(sulfadimethoxine/ormetoprim)Each tablet contains 500 mg sulfadimethoxine
and 100 mg ormetoprim.For use in dogs only
Not for Human Use
Keep Out of Reach of Children
Caution: Federal law restricts this drug to use
by or on the order of a licensed veterinarian.100 tablets
NADA #100-929, Approved by FDAzoetis

-
PRINCIPAL DISPLAY PANEL - 1200 mg Tablet Bottle Label
PRIMOR®
1200
(sulfadimethoxine/
ormetoprim)Each tablet contains 1000 mg
sulfadimethoxine and
200 mg ormetoprim.For use in dogs only
Not for Human Use
Keep Out of Reach of Children
Caution: Federal law restricts this
drug to use by or on the order of
a licensed veterinarian.100 tablets
NADA #100-929, Approved by FDAzoetis
-
INGREDIENTS AND APPEARANCE
PRIMOR
sulfadimethoxine and ormetoprim tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 54771-8440 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength sulfadimethoxine (UNII: 30CPC5LDEX) (sulfadimethoxine - UNII:30CPC5LDEX) sulfadimethoxine 100 mg ormetoprim (UNII: M3EFS94984) (ormetoprim - UNII:M3EFS94984) ormetoprim 20 mg Product Characteristics Color BLUE Score 2 pieces Shape OVAL Size 10mm Flavor Imprint Code Primor;120 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54771-8440-1 100 in 1 BOTTLE 2 NDC: 54771-8440-2 1000 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA100929 11/24/1989 PRIMOR
sulfadimethoxine and ormetoprim tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 54771-8441 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength sulfadimethoxine (UNII: 30CPC5LDEX) (sulfadimethoxine - UNII:30CPC5LDEX) sulfadimethoxine 200 mg ormetoprim (UNII: M3EFS94984) (ormetoprim - UNII:M3EFS94984) ormetoprim 40 mg Product Characteristics Color BLUE Score 2 pieces Shape OVAL Size 13mm Flavor Imprint Code Primor;240 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54771-8441-1 100 in 1 BOTTLE 2 NDC: 54771-8441-2 500 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA100929 11/24/1989 PRIMOR
sulfadimethoxine and ormetoprim tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 54771-8442 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength sulfadimethoxine (UNII: 30CPC5LDEX) (sulfadimethoxine - UNII:30CPC5LDEX) sulfadimethoxine 500 mg ormetoprim (UNII: M3EFS94984) (ormetoprim - UNII:M3EFS94984) ormetoprim 100 mg Product Characteristics Color BLUE Score 2 pieces Shape OVAL Size 18mm Flavor Imprint Code Primor;600 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54771-8442-1 100 in 1 BOTTLE 2 NDC: 54771-8442-2 250 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA100929 11/24/1989 PRIMOR
sulfadimethoxine and ormetoprim tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 54771-8444 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength sulfadimethoxine (UNII: 30CPC5LDEX) (sulfadimethoxine - UNII:30CPC5LDEX) sulfadimethoxine 1000 mg ormetoprim (UNII: M3EFS94984) (ormetoprim - UNII:M3EFS94984) ormetoprim 200 mg Product Characteristics Color BLUE Score 2 pieces Shape OVAL (biconvex with flat sides) Size 22mm Flavor Imprint Code Primor;1200 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54771-8444-1 100 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA100929 11/24/1989 Labeler - Zoetis Inc. (828851555) Establishment Name Address ID/FEI Business Operations Zoetis LLC 039055157 MANUFACTURE Establishment Name Address ID/FEI Business Operations EMCURE PHARMACEUTICALS LIMITED 862602830 MANUFACTURE Establishment Name Address ID/FEI Business Operations Shanghai Zhongxi Sunve Pharmaceutical Co. Ltd 544646342 API MANUFACTURE
Trademark Results [Primor]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PRIMOR 86840733 not registered Live/Pending |
Ganir (1992) Ltd. 2015-12-07 |
 PRIMOR 86527009 4989603 Live/Registered |
Comep USA, Inc. 2015-02-06 |
 PRIMOR 85957188 4606647 Live/Registered |
First Choice Bank 2013-06-11 |
 PRIMOR 79188954 5202241 Live/Registered |
KUHN-AUDUREAU SA 2016-04-05 |
 PRIMOR 79035662 3385766 Dead/Cancelled |
CONSERVERA GALLEGA, S.A. 2007-01-02 |
 PRIMOR 78937106 3307266 Dead/Cancelled |
Cerda, Enrique Fonseca 2006-07-25 |
 PRIMOR 78285693 3071761 Live/Registered |
Creativa Interiors LLC 2003-08-11 |
 PRIMOR 78285688 3071760 Live/Registered |
Creativa Interiors LLC 2003-08-11 |
 PRIMOR 78285650 3004969 Live/Registered |
Creativa Interiors LLC 2003-08-11 |
 PRIMOR 78267518 3068985 Live/Registered |
Creativa Interiors LLC 2003-06-26 |
 PRIMOR 78267255 3090938 Live/Registered |
Creativa Interiors LLC 2003-06-26 |
 PRIMOR 78267253 3093827 Live/Registered |
Creativa Interiors LLC 2003-06-26 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.