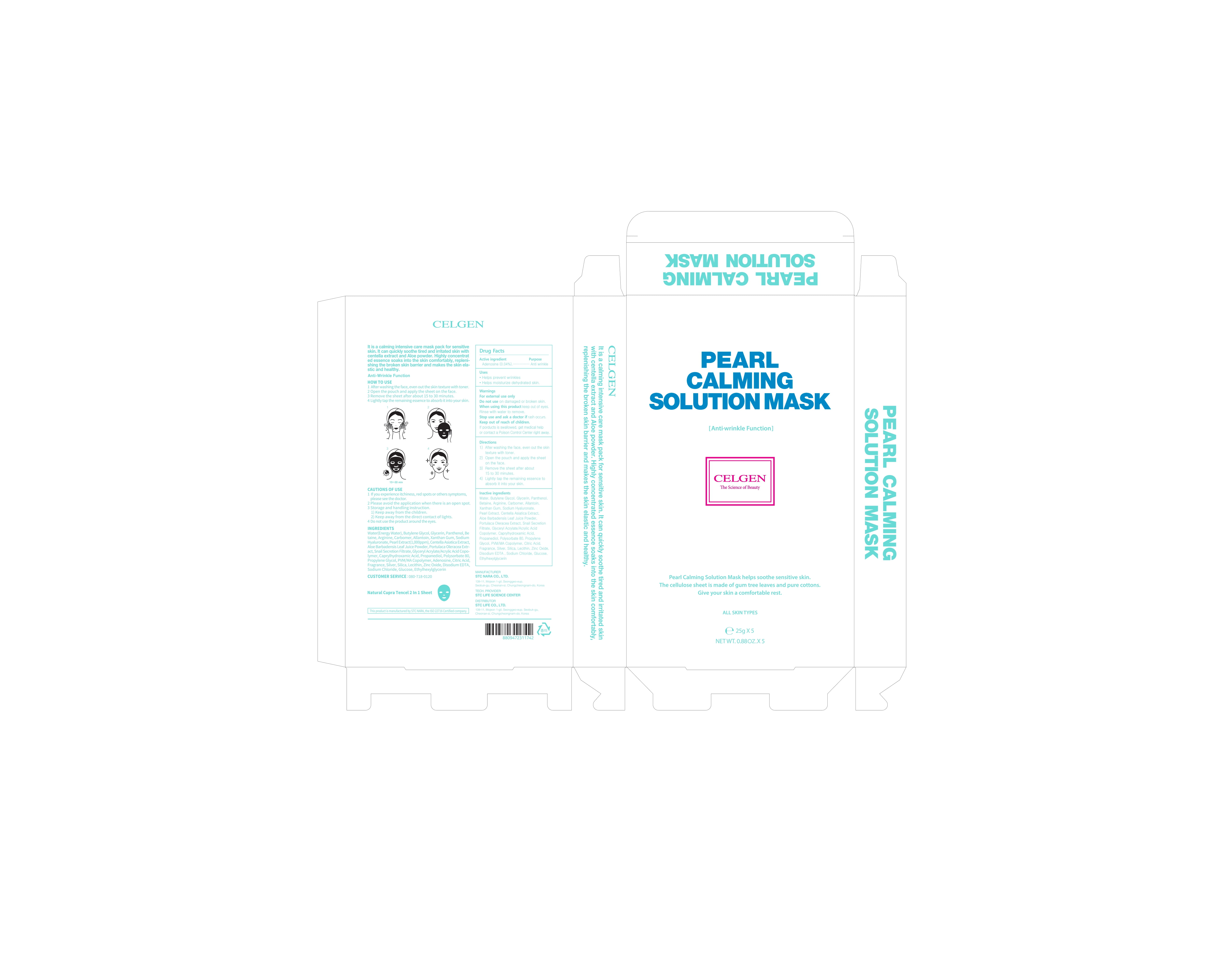
76731-212 CELGEN Pearl Calming Solution Mask
CELGEN Pearl Calming Solution Mask by
Drug Labeling and Warnings
CELGEN Pearl Calming Solution Mask by is a Otc medication manufactured, distributed, or labeled by BBHC CO., LTD, STC Nara Co., Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CELGEN PEARL CALMING SOLUTION MASK- adenosine lotion
BBHC CO., LTD
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
76731-212 CELGEN Pearl Calming Solution Mask
Keep out of reach of children.
If porducts is swallowed, get medical help
or contact a Poison Control Center right away.
Directions
1) After washing the face, even out the skin texture with toner.
2) Open the pouch and apply the sheet on the face.
3) Remove the sheet after about 15 to 30 minutes.
4) Lightly tap the remaining essence to absorb it into your skin.
Inactive ingredients
Water, Butylene Glycol, Glycerin, Panthenol, Betaine, Arginine, Carbomer, Allantoin, Xanthan Gum, Sodium Hyaluronate, Pearl Extract, Centella Asiatica Extract, Aloe Barbadensis Leaf Juice Powder, Portulaca Oleracea Extract, Snail Secretion Filtrate, Glyceryl crylate/Acrylic Acid Copolymer, Caprylhydroxamic Acid, Propanediol, Polysorbate 80, Propylene Glycol, PVM/MA Copolymer, Citric Acid, Fragrance, Silver, Silica, Lecithin, Zinc Oxide, Disodium EDTA , Sodium Chloride, Glucose, Ethylhexylglycerin
| CELGEN PEARL CALMING SOLUTION MASK
adenosine lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - BBHC CO., LTD (689522401) |
| Registrant - STC Nara Co., Ltd (689135085) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| STC Nara Co., Ltd | 689135085 | manufacture(76731-212) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.