DRAINPAR (solidago virgaurea, trifolium pratense, propolis, hepar suis, kidney (suis), lymph node (suis), berberis vulgaris, bryonia (alba), ceanothus americanus, chelidonium majus, nux vomica, sarsaparilla- smilax regelii, taraxacum officinale liquid
Drainpar by
Drug Labeling and Warnings
Drainpar by is a Homeopathic medication manufactured, distributed, or labeled by Energique, Inc., Apotheca Company. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
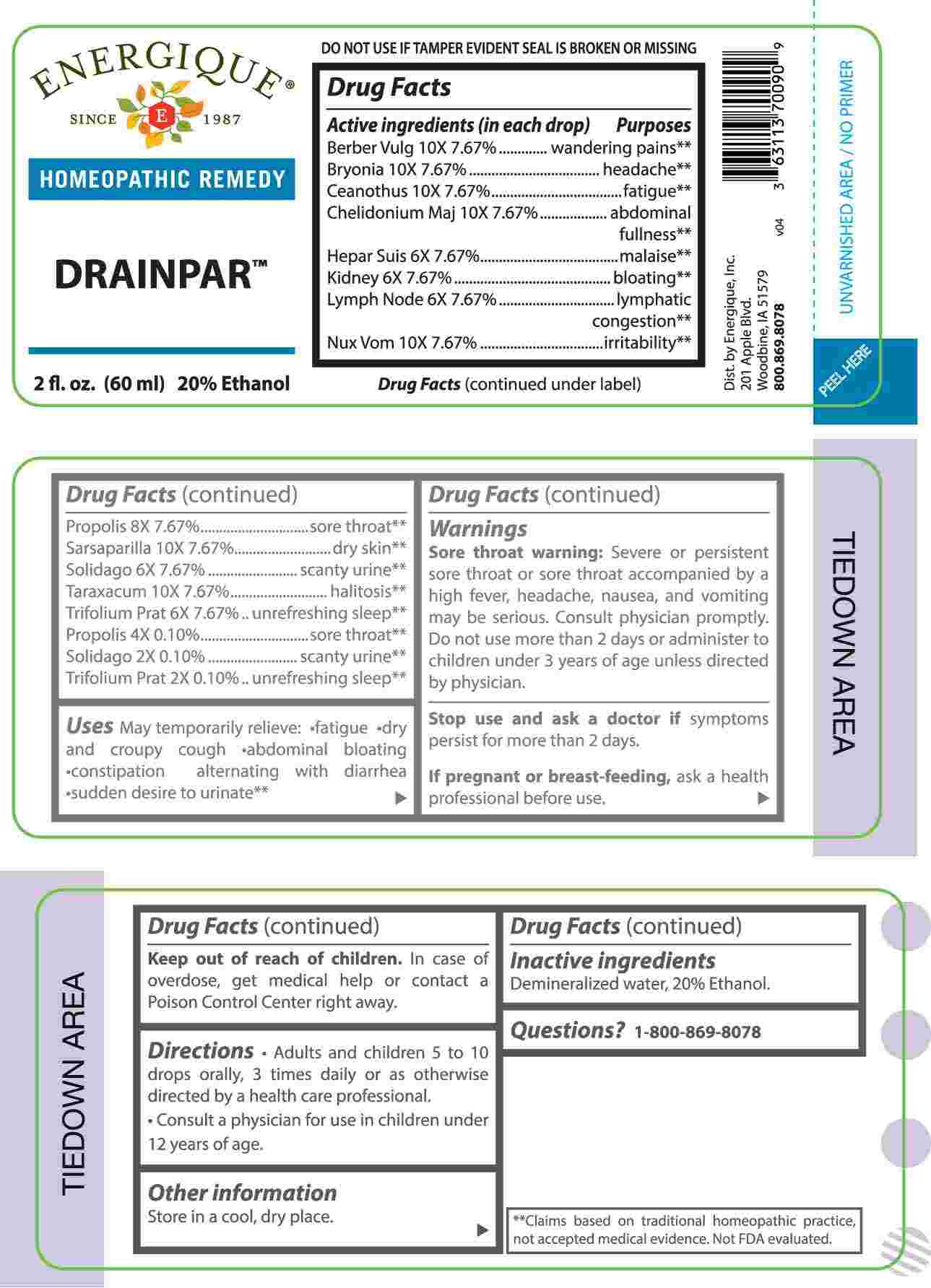
ACTIVE INGREDIENTS:
(in each drop) Berberis Vulgaris 10X 7.67%, Bryonia 10X 7.67%, Ceanothus Americanus 10X 7.67%, Chelidonium Majus 10X 7.67%, Hepar Suis 6X 7.67%, Kidney 6X 7.67%, Lymph Node 6X 7.67%, Nux Vomica 10X 7.67%, Propolis 8X 7.67%, Sarsaparilla 10X 7.67%, Solidago Virgaurea 6X 7.67%, Taraxacum Officinale 10X 7.67%, Trifolium Pratense 6X 7.67%, Propolis 4X 0.10%, Solidago Virgaurea 2X 0.10%, Trifolium Pratense 2X 0.10%
-
PURPOSE:
Berberis Vulgaris - wandering pain,** Bryonia - headache,** Ceanothus Americanus - fatigue,** Chelidonium Majus – abdominal fullness,** Hepar Suis - malaise,** Kidney - bloating,** Lymph Node – lymphatic congestion ,** Nux Vomica - irritability,** Propolis – sore throat,** Sarsaparilla – dry skin,** Solidago Virgaurea – scanty urine,** Taraxacum Officinale - halitosis,** Trifolium Pratense – unrefreshing sleep,**
- USES:
-
WARNINGS:
Sore throat warning: Severe or persistent sore throat or sore throat accompanied by a high fever, headache, nausea, and vomiting may be serious. Consult physician promptly. Do not use more than 2 days or administer to children under 3 years of age unless directed by physician.
Stop use and ask a doctor if symptoms persist for more than 2 days.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
DO NOT USE IF TAMPER EVIDENT SEAL IS BROKEN OR MISSING
Store in a cool, dry place.
- KEEP OUT OF REACH OF CHILDREN:
- DIRECTIONS:
- INACTIVE INGREDIENTS:
- QUESTIONS:
- PACKAGE LABEL DISPLAY:
-
INGREDIENTS AND APPEARANCE
DRAINPAR
solidago virgaurea, trifolium pratense, propolis, hepar suis, kidney (suis), lymph node (suis), berberis vulgaris, bryonia (alba), ceanothus americanus, chelidonium majus, nux vomica, sarsaparilla (smilax regelii), taraxacum officinale liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 44911-0731 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SOLIDAGO VIRGAUREA FLOWERING TOP (UNII: 5405K23S50) (SOLIDAGO VIRGAUREA FLOWERING TOP - UNII:5405K23S50) SOLIDAGO VIRGAUREA FLOWERING TOP 2 [hp_X] in 1 mL TRIFOLIUM PRATENSE FLOWER (UNII: 4JS0838828) (TRIFOLIUM PRATENSE (CLOVER) FLOWER - UNII:4JS0838828) TRIFOLIUM PRATENSE FLOWER 2 [hp_X] in 1 mL PROPOLIS WAX (UNII: 6Y8XYV2NOF) (PROPOLIS CERA - UNII:6Y8XYV2NOF) PROPOLIS WAX 4 [hp_X] in 1 mL PORK LIVER (UNII: 6EC706HI7F) (PORK LIVER - UNII:6EC706HI7F) PORK LIVER 6 [hp_X] in 1 mL PORK KIDNEY (UNII: X7BCI5P86H) (PORK KIDNEY - UNII:X7BCI5P86H) PORK KIDNEY 6 [hp_X] in 1 mL SUS SCROFA LYMPH (UNII: 33A7VYU29L) (SUS SCROFA LYMPH - UNII:33A7VYU29L) SUS SCROFA LYMPH 6 [hp_X] in 1 mL BERBERIS VULGARIS ROOT BARK (UNII: 1TH8Q20J0U) (BERBERIS VULGARIS ROOT BARK - UNII:1TH8Q20J0U) BERBERIS VULGARIS ROOT BARK 10 [hp_X] in 1 mL BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 10 [hp_X] in 1 mL CEANOTHUS AMERICANUS LEAF (UNII: 25B1Y14T8N) (CEANOTHUS AMERICANUS LEAF - UNII:25B1Y14T8N) CEANOTHUS AMERICANUS LEAF 10 [hp_X] in 1 mL CHELIDONIUM MAJUS WHOLE (UNII: 7E889U5RNN) (CHELIDONIUM MAJUS - UNII:7E889U5RNN) CHELIDONIUM MAJUS WHOLE 10 [hp_X] in 1 mL STRYCHNOS NUX-VOMICA SEED (UNII: 269XH13919) (STRYCHNOS NUX-VOMICA SEED - UNII:269XH13919) STRYCHNOS NUX-VOMICA SEED 10 [hp_X] in 1 mL SMILAX ORNATA ROOT (UNII: 2H1576D5WG) (SARSAPARILLA - UNII:2H1576D5WG) SMILAX ORNATA ROOT 10 [hp_X] in 1 mL TARAXACUM OFFICINALE (UNII: 39981FM375) (TARAXACUM OFFICINALE - UNII:39981FM375) TARAXACUM OFFICINALE 10 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 44911-0731-1 60 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 03/18/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 03/18/2025 Labeler - Energique, Inc. (789886132) Registrant - Apotheca Company (844330915) Establishment Name Address ID/FEI Business Operations Apotheca Company 844330915 manufacture(44911-0731) , api manufacture(44911-0731) , label(44911-0731) , pack(44911-0731)
© 2025 FDA.report
This site is not affiliated with or endorsed by the FDA.