SODIUM SULFACETAMIDE gel
Sodium Sulfacetamide by
Drug Labeling and Warnings
Sodium Sulfacetamide by is a Prescription medication manufactured, distributed, or labeled by Acella Pharmaceuticals, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
DESCRIPTION: Each mL of SODIUM SULFACETAMIDE 10% CLEANSING GEL contains 100 mg of sodium sulfacetamide, USP in a formulation containing Citric acid, Cocamidopropyl Betaine, Disodium EDTA, Glycerin, Glyceryl Stearate, Methylparaben, PEG-6 Caprylic/Capric Glycerides, PEG-60 Almond Glycerides, PEG-150 Pentaerythrityl Tetrastearate, Polysorbate 60, Sodium Lauryl Sulfate, Sodium Thiosulfate, Water, Xanthan Gum.
Sodium sulfacetamide is C 8H 9N 2NaO 3S·H 2O with molecular weight of 254.24. Chemically, it is N-[(4-aminophenyl)sulfonyl]-acetamide, monosodium salt, monohydrate. The structural formula is:
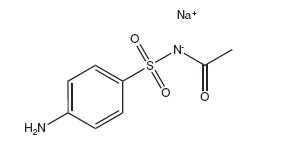
Sodium sulfacetamide is an odorless, white, crystalline powder with a bitter taste. It is freely soluble in water, sparingly soluble in alcohol, while practically insoluble in benzene, in chloroform and in ether.
-
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGY: Sodium sulfacetamide exerts a bacteriostatic effect against sulfonamide sensitive Gram-positive and Gram-negative microorganisms commonly isolated from secondary cutaneous pyogenic infections. It acts by restricting the synthesis of folic acid required by bacteria for growth, by its competition with para-aminobenzoic acid. There is no clinical data available on the degree and rate of systemic absorption of SODIUM SULFACETAMIDE 10% CLEANSING GEL when applied to the skin or scalp. However, significant absorption of sodium sulfacetamide through the skin has been reported. The following in vitro data is available but the clinical significance is unknown. Organisms that show susceptibility to sodium sulfacetamide are: Streptococci, Staphylococci, E. coli, Klebsiella pneumoniae, Pseudomonas pyocyanea, Salmonella species, Proteus vulgaris, Nocardia and Actinomyces.
-
INDICATIONS & USAGE
INDICATIONS AND USAGE: SODIUM SULFACETAMIDE 10% CLEANSING GEL is intended for topical application in the following scaling dermatoses: seborrheic dermatitis and seborrhea sicca (dandruff). It also is indicated for the treatment of secondary bacterial infections of the skin due to organisms susceptible to sulfonamides.
- CONTRAINDICATIONS
-
WARNINGS
WARNINGS: Sulfonamides are known to cause Stevens-Johnson syndrome in hypersensitive individuals. Stevens-Johnson syndrome also has been reported following the use of sodium sulfacetamide topically. Cases of drug-induced systemic lupus erythematosus from topical sulfacetamide also have been reported. In one of these cases, there was a fatal outcome. KEEP OUT OF THE REACH OF CHILDREN.
-
WARNINGS AND PRECAUTIONS
PRECAUTIONS:
FOR EXTERNAL USE ONLY. NOT FOR OPHTHALMIC USE.
General: Nonsusceptible organisms, including fungi, may proliferate with the use of this preparation. Hypersensitivity reactions may recur when a sulfonamide is readministered, irrespective of the route of administration, and cross hypersensitivity between different sulfonamides may occur. If SODIUM SULFACETAMIDE 10% CLEANSING GEL produces signs of hypersensitivity or other untoward reactions, discontinue use of the preparation. Systemic absorption of topical sulfonamides is greater following application to large, infected, abraded, denuded or severely burned areas. Under these circumstances, any of the adverse effects produced by the systemic administration of these agents could potentially occur, and appropriate observations and laboratory determinations should be performed.
-
INFORMATION FOR PATIENTS
Information for Patients: Patients should discontinue SODIUM SULFACETAMIDE 10% CLEANSING GEL if the condition becomes worse, or if a rash develops in the area being treated or elsewhere. SODIUM SULFACETAMIDE 10% CLEANSING GEL also should be discontinued promptly and the physician notified if any arthritis, fever or sores in the mouth develop.
- DRUG INTERACTIONS
- SPL UNCLASSIFIED SECTION
-
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
Carcinogenesis, Mutagenesis and Impairment of Fertility: Long-term animal studies for carcinogenic potential have not been performed on SODIUM SULFACETAMIDE 10% CLEANSING GEL to date. Studies on reproduction and fertility also have not been performed. Chromosomal nondisjunction has been reported in the yeast, Saccharomyces cerevisiae, following application of sodium sulfacetamide. The significance of this finding to the topical use of sodium sulfacetamide in the human is unknown.
-
PREGNANCY
Pregnancy: Category C. Animal reproduction studies have not been conducted with SODIUM SULFACETAMIDE 10% CLEANSING GEL. It is also not known whether SODIUM SULFACETAMIDE 10% CLEANSING GEL can affect reproduction capacity or cause fetal harm when administered to a pregnant woman. SODIUM SULFACETAMIDE 10% CLEANSING GEL should be used by a pregnant woman only if clearly needed or when potential benefits outweigh potential hazards to the fetus.
- NURSING MOTHERS
- PEDIATRIC USE
-
ADVERSE REACTIONS
ADVERSE REACTIONS: Reports of irritation and hypersensitivity to sodium sulfacetamide are uncommon. The following adverse reactions, reported after administration of sterile ophthalmic sodium sulfacetamide, are noteworthy: instances of Stevens-Johnson syndrome and instances of local hypersensitivity which progressed to a syndrome resembling systemic lupus erythematosus; in one case a fatal outcome was reported (see WARNINGS). Call your doctor for medical advice about side effects.
-
OVERDOSAGE
OVERDOSAGE: The oral LD50 of sulfacetamide in mice is 16.5 g/kg. In the event of overdosage, emergency treatment should be started immediately.
To report SUSPECTED ADVERSE REACTIONS, contact the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Manifestations: Overdosage may cause nausea and vomiting. Large oral overdosage may cause hematuria, crystalluria and renal shutdown due to the precipitation of sulfa crystals in the renal tubules and the urinary tract. For treatment, contact your local Poison Control Center or your doctor.
-
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION: Seborrheic dermatitis including seborrhea sicca - Wash affected areas twice daily (morning and evening), or as directed by your physician. Avoid contact with eyes or mucous membranes. Wet skin and liberally apply to areas to be cleansed, massage gently into skin working into a full lather, rinse thoroughly, pat dry and repeat after 10 to 20 seconds. Rinsing with plain water will remove any excess medication. Repeat application as described for 8 to 10 days. lf skin dryness occurs it may be controlled by rinsing cleanser off sooner or using less frequently. Regular shampooing following SODIUM SULFACETAMIDE 10% CLEANSING GEL is not necessary, but the hair should be shampooed at least once a week. As the condition subsides, the interval between applications may be lengthened. Applications once or twice weekly or every other week may prevent recurrence. Should the condition recur after stopping therapy, the application of SODIUM SULFACETAMIDE 10% CLEANSING GEL should be reinitiated as at the beginning of treatment.
Secondary cutaneous bacterial infections - Wet skin and liberally apply to areas to be cleansed, massage gently into skin for 10 to 20 seconds working into a full lather, rinse thoroughly and pat dry. Rinsing with plain water will remove any excess medication. Repeat application as described for 8 to 10 days. If skin dryness occurs it may be controlled by rinsing cleanser off sooner or using less often.
-
HOW SUPPLIED:
SODIUM SULFACETAMIDE 10% CLEANSING GEL is available in a 12 fl. oz. (355 mL) bottle, NDC: 42192-146-12.
- STORAGE AND HANDLING
-
SPL UNCLASSIFIED SECTION
Note: Protect from freezing and excessive heat. The product may tend to darken slightly on storage. Slight discoloration does not impair the efficacy or safety of the product. Keep container or packet tightly closed.
A slight yellowish discoloration may occur on occasion when an excessive amount of the product is used and comes in contact with white fabrics. This discoloration is easily removed by normal laundering; bleaching is not necessary.
All prescription substitutions and/or recommendations using this product shall be made subject to state and federal statutes as applicable. Please NOTE: This is not an Orange Book product and has not been subjected to FDA therapeutic or other equivalency testing. No representation is made as to generic status or bioequivalency. Each person recommending a prescription substitution using this product shall make such recommendation based on his/her professional knowledge and opinion, upon evaluating the active ingredients, inactive ingredients, excipients and chemical information provided herein.
Manufactured for:
Acella Pharmaceuticals, LLC
11675 Great Oaks Way
Alpharetta, GA 30022
1-800-541-4802Rev. 0914
-
PACKAGE LABEL - 12 fl. oz. (355 mL) bottle
NDC: 42192-146-12
Sodium Sulfacetamide
10% Cleansing GelRx Only
For External Use Only
12 fl. oz. (355 mL)
Acella
PHARMACEUTICALS, LLC
-
INGREDIENTS AND APPEARANCE
SODIUM SULFACETAMIDE
sodium sulfacetamide gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 42192-146 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULFACETAMIDE SODIUM (UNII: 4NRT660KJQ) (SULFACETAMIDE - UNII:4965G3J0F5) SULFACETAMIDE SODIUM 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) METHYLPARABEN (UNII: A2I8C7HI9T) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PENTAERYTHRITYL TETRASTEARATE (UNII: W9Q3DZS0EG) POLYSORBATE 60 (UNII: CAL22UVI4M) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SODIUM THIOSULFATE (UNII: HX1032V43M) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) PEG-60 ALMOND GLYCERIDES (UNII: 4Y0E651N0F) PEG-6 CAPRYLIC/CAPRIC GLYCERIDES (UNII: GO50W2HWO8) PEG-150 PENTAERYTHRITYL TETRASTEARATE (UNII: 8L4OOQ76AM) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42192-146-12 355 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/10/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/10/2014 Labeler - Acella Pharmaceuticals, LLC (825380939) Establishment Name Address ID/FEI Business Operations Acella Pharmaceuticals, LLC 825380939 manufacture(42192-146)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.