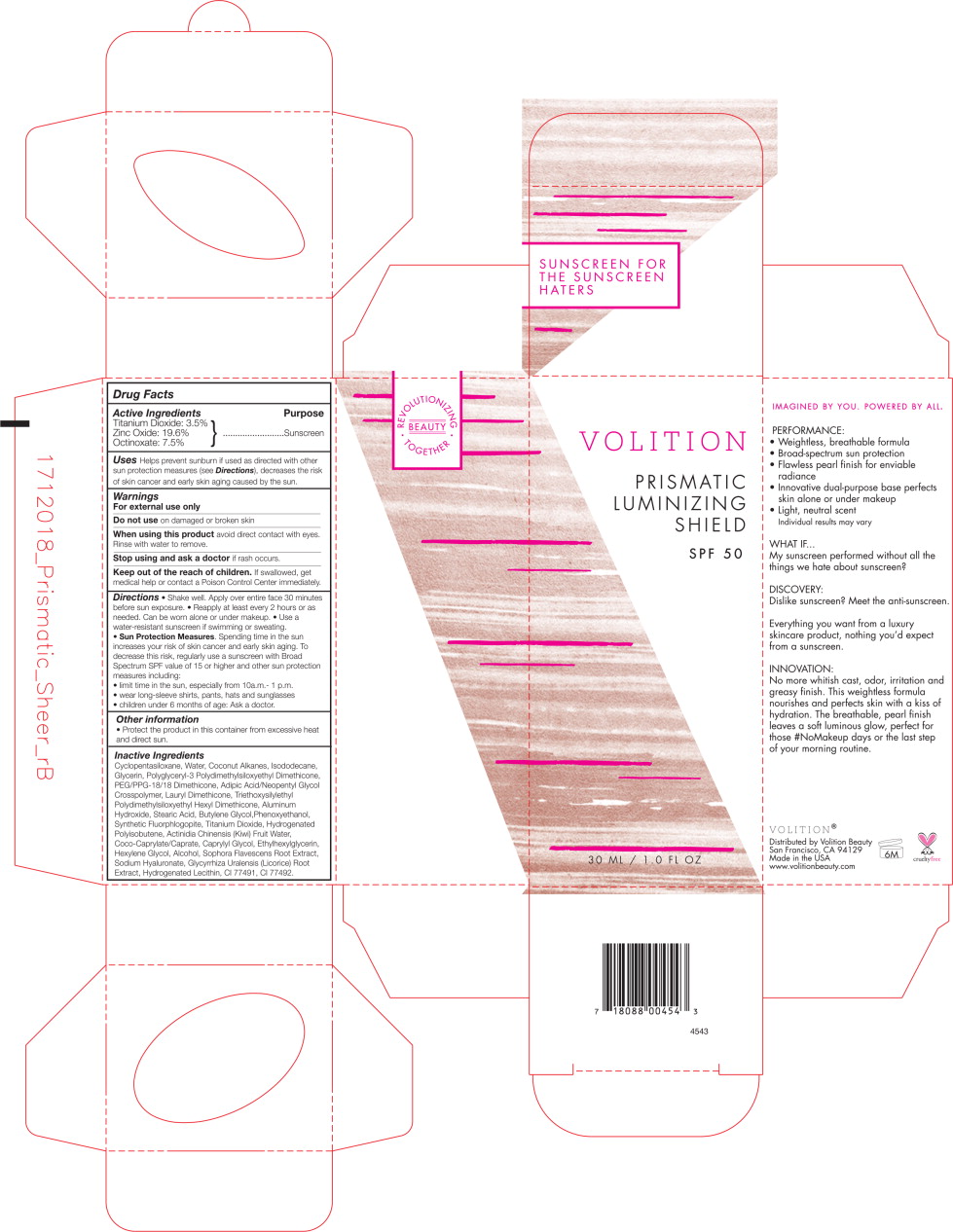
Prismatic Protectant SPF50 by Volition Beauty / PhytogenX, Inc. Drug Facts
Prismatic Protectant SPF50 by
Drug Labeling and Warnings
Prismatic Protectant SPF50 by is a Otc medication manufactured, distributed, or labeled by Volition Beauty, PhytogenX, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
PRISMATIC PROTECTANT SPF50- zinc oxide, titanium dioxide, octinoxate lotion
Volition Beauty
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Uses
Helps prevent sunburn if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Directions
- Shake well. Apply over entire face 30 minutes before sun exposure.
- Reapply at least every 2 hours or as needed. Can be worn alone or under makeup.
- Use a water-resistant sunscreen if swimming or sweating.
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 1 p.m.
- wear long-sleeve shirts, pants, hats and sunglasses
- children under 6 months of age: Ask a doctor.
Inactive Ingredients
Cyclopentasiloxane, Water, Coconut Alkanes, Isododecane, Glycerin, Polyglyceryl-3 Polydimethylsiloxyethyl Dimethicone, PEG/PPG-18/18 Dimethicone, Adipic Acid/Neopentyl Glycol Crosspolymer, Lauryl Dimethicone, Triethoxysilylethyl Polydimethylsiloxyethyl Hexyl Dimethicone, Aluminum Hydroxide, Stearic Acid, Butylene Glycol, Phenoxyethanol, Synthetic Fluorphlogopite, Titanium Dioxide, Hydrogenated Polyisobuetene, Actinidia Chinensis (Kiwi) Fruit Water, Coco-Caprylate/Caprate, Caprylyl Glycol, Ethylhexylglycerin, Hexylene Glycol, Alcohol, Sophora Flavescens Root Extract, Sodium Hyaluronate, Glycyrrhiza Uralensis (Licorice) Root Extract, Hydrogenated Lecithin, CI 77491, CI 77492.
Principal Display Panel - Prismatic Protectant SPF50 Bottle Label
VOLITION
PRISMATIC
LUMINIZING
SHIELD
SPF50
30 ML/1.0 FL OZ
| PRISMATIC PROTECTANT SPF50
zinc oxide, titanium dioxide, octinoxate lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Volition Beauty (023668513) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| PhytogenX, Inc. | 624386772 | manufacture(72577-326) | |