SUSTAIN III- sulfamethazine tablet
Sustain III by
Drug Labeling and Warnings
Sustain III by is a Animal medication manufactured, distributed, or labeled by Bimeda, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DOSAGE & ADMINISTRATION
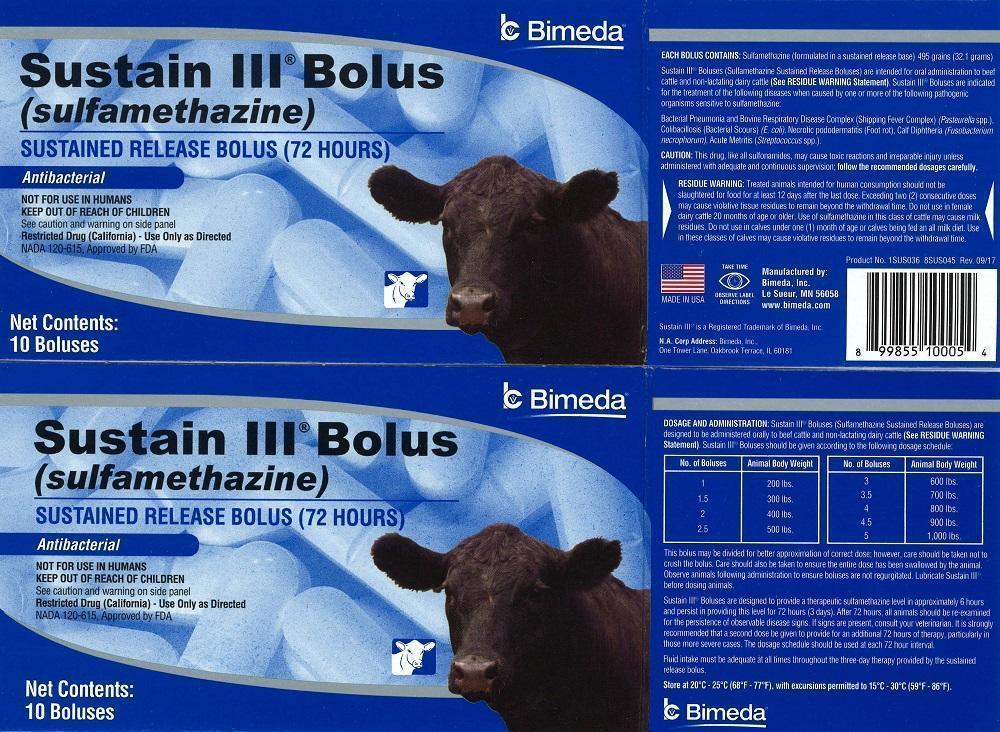
DOSAGE AND ADMINISTRATION: Sustain III® Boluses (Sulfamethazine Sustained Release Boluses) are designed to be administered orally to beef cattle and non-lactating dairy cattle (See RESIDUE WARNING Statement). Sustain III Boluses should be given according to the following dosage schedule:
No. of Boluses Animal Body Weight
1 200 lbs.
1.5 300 lbs.
2 400 lbs.
2.5 500 lbs.
3 600 lbs.
3.5 700 lbs.
4 800 lbs.
4.5 950 lbs.
5 1,000 lbs.This bolus may be divided for better approximation of correct dose; however, care should be taken not to crush the bolus. Care should also be taken to ensure the entire dose has been swallowed by the animal. Observe animals following administration to ensure boluses are not regurgitated. Lubricate Sustain III® before dosing animals.
Sustain III® Boluses are designed to provide a therapeutic sulfamethazine level in approximately 6 hours and persist in providing this level for 72 hours (3 days). After 72 hours, all animals should be re-examined for persistence of observable disease signs. If signs are present, consult your veterinarian. It is strongly recommended that a second dose be given to provide for an additional 72 hours of therapy, particularly in those more severe cases. The dosage schedule should be used at each 72-hour interval.
Fluid intake must be adequate at all times throughout the three-day therapy provided by the sustained release bolus.
- STORAGE AND HANDLING
-
DESCRIPTION
EACH BOLUS CONTAINS: Sulfamethazine (formulated in a sustained release base) 495 grains (32.1 grams)
Sustain III® Boluses (Sulfamethazine Sustained Release Boluses) are intended for oral administration to beef cattle and non-lactating dairy cattle (See RESIDE WARNING Statement). Sustain III® Boluses are indicated for the treatment of the following diseases when caused by one or more of the following pathogenic organisms sensitive to sulfamethazine:
Bacterial Pneumonia and Bovine Respiratory Disease Complex (Shipping Fever Complex) (Pasteurella spp.).
Colibacillosis (Bacterial Scours) (E. coli), Necrotic pododermatitis (Foot rot), Calf Diphtheria (Fusobacterium necrophorum), Acute Metritis (Streptococcus spp.).
- PRECAUTIONS
-
RESIDUE WARNING
RESIDUE WARNING: Treated animals intended for human consumption should not be slaughtered for food for at least 12 days after the last dose. Exceeding two (2) consecutive doses may cause violative tissue residues to remain beyond the withdrawal time. Do not use in female dairy cattle 20 months of age or older. Use of sulfamethazine in this class of cattle may cause milk residues. Do not use in calves under one (1) month of age or calves being fed an all milk diet. Use in these classes of calves may cause violative residues to remain beyond the withdrawal time.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SUSTAIN III
sulfamethazine tabletProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC: 61133-1331 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sulfamethazine (UNII: 48U51W007F) (SULFAMETHAZINE - UNII:48U51W007F) Sulfamethazine 32.1 g Product Characteristics Color white Score 2 pieces Shape OVAL Size 1935mm Flavor Imprint Code NA Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 61133-1331-2 100 in 1 CARTON 2 NDC: 61133-1331-1 10 in 1 CARTON 3 NDC: 61133-1331-3 50 in 1 CARTON Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA120615 07/14/1984 Labeler - Bimeda, Inc. (060492923) Registrant - Bimeda, Inc. (060492923) Establishment Name Address ID/FEI Business Operations Bimeda, Inc. 060492923 manufacture
Trademark Results [Sustain III]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SUSTAIN III 74181716 1732411 Live/Registered |
BIMEDA INC. 1991-07-02 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.